- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
You are on the right track by first counting the number of carbons. Actually, both C AND D start with seven carbons, so we eliminate both C and D.
Therefore, we focus on A and B.
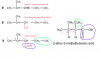
Step 1: Replace the Br atom with CN
Step 2: hydrolyze the CN to COOH
Generally, the smaller the atoms orbitals that overlap, the stronger the bonds. This trend is seem by the bond strength decreasing from Cl-Cl>Br-Br>I-I.
H-F being stronger than H-Cl can also be explained in that manner.
However although F atoms are really small and thus expected to have the most overlap it is actually an exception to the general rule. This is because the two nuclei (each 9 protons) will repel each other if there are too close together (lone pair of electrons will also repel each other). Thus the bond length ends up longer than expected.
So how do we decide between A and B?
But why is that only with F-F? I mean H-F bond also consists of smaller atomic orbitals that overlap,no?