- Messages
- 15
- Reaction score
- 10
- Points
- 13
The reaction with bromineRight thankyou
btw can u tell me the difference in the reaction of benzene and methyl benzene with br2
The reaction between benzene and bromine takes place with the help of a catalyst such as AlBr3 (if you are reacting with Cl2 then the catalyst will be AlCl3)
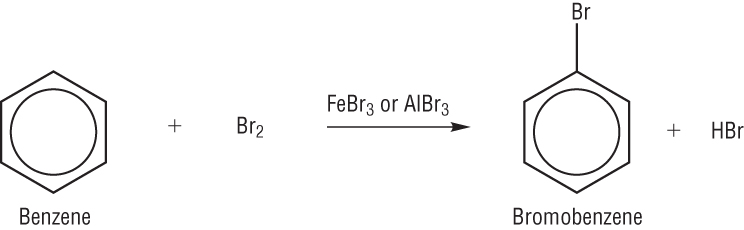

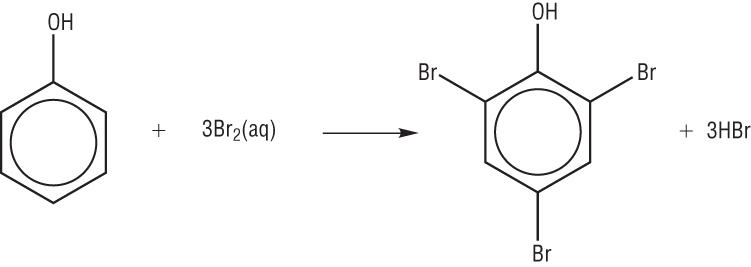
OH and CH3 are electron releasing (electron withdrawing) groups, this means that they are 2 ,4 directing group. As in the above diagram the Br2 is in excess so it will take all 3 positions : 2,4,6. If in limited quantity then it will occupy only 2 position.
See the Untitled.png which i have attached. Once you have gone through it you wont have any problem regarding positioning of atoms/molecules e.g Br2 etc
Hope this helps.



