- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
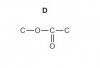
3c) You'll have to draw a double hump diagram for this. It's essentially the same as the simpler energy diagram except we break the single hump into 2 to represent the intermediate steps. There should be two peaks and the first one should be higher than the second. If you recall free radical substitution mechanism you know the intermediate steps. The first hump represents the formation of methyl free radical and HCl and the second represents the chloromethane formation. The reason the second hump is lower is because the reaction can only proceed as long as the energy barrier for the following step is less then the preceding one. The activation energy is labeled for the first hump.http://maxpapers.com/syllabus-materials/chemistry-9701-a-level/attachment/9701_w09_qp_21/
Xylferion Please can you answer 3 part c?
-lilcloud!