- Messages
- 479
- Reaction score
- 1,374
- Points
- 153
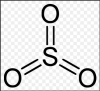
thankyou!2Mg(NO3)2 ----> 2MgO + 4NO2 + O2
The equation shows us that for every 4 moles of NO2 released, 1 mole of O2 is released.
Mass of NO2: (14 + 16x2) x 4 = 46 x 4 = 184 grams
Mass of O2: (16x2)x1 = 32 grams.
184/32 = 5.75 = 1/0.174 = A!