- Messages
- 58
- Reaction score
- 21
- Points
- 8
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Ecell = 0.76V
I always used trial and error method for this kindda questions.The correct answer here is B. Some one explain whyView attachment 58111
Niice thanks !I always used trial and error method for this kindda questions.
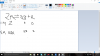
For option B:~
View attachment 58116
Sum of moles at equilibrium = 2 - 2x + 2x + x = 2 + x
can u kindly give me any notes u have for physics and chemistry ial notes ASAPI have notes for chemistry unit 3 and physics unit 3, you are doing edexcel IAL correct? If so, just give me your email so i can send you the files
Didn't get the tag.View attachment 58131
25 a and b
I guess I know the answer, but I am not sure if I am 100% correct.
a) From all three Cr2+ has least positive E(std) value. Hence it needs the strongest reducing agent (more -ve E(std) value agent) to reduce it.
b) From all three Ag has most posivtive E(std) value. Hence it needs the strongest oxidising agent (more +ve E(std) value agent) to oxidise it.
M I corrct? If not then do correct me. Thanks.
Metanoia
DarkEclipse
Dark Destination
awesomaholic101
FranticAmaze
My Name
Those answers better be right .. Coz I would say that too and I don't want to be wrongDidn't get the tag.
I think you're answers are right.
Doesn't the book have answers at the back?
awesomaholic101 FranticAmaze Midnight dream Lola_sweet What do you say?
View attachment 58131
25 a and b
I guess I know the answer, but I am not sure if I am 100% correct.
a) From all three Cr2+ has least positive E(std) value. Hence it needs the strongest reducing agent (more -ve E(std) value agent) to reduce it.
b) From all three Ag has most posivtive E(std) value. Hence it needs the strongest oxidising agent (more +ve E(std) value agent) to oxidise it.
M I corrct? If not then do correct me. Thanks.
Metanoia
DarkEclipse
Dark Destination
awesomaholic101
FranticAmaze
My Name
THanks....Yes. That is correct. You can also use O level chemistry to check your answer- in decreasing reactivity of metals, Chromium, Iron and Silver.
So the most stable ion is Cr2+ and the most stable metal is Ag.
They will require the strongest reducing agent and oxidizing agent respectively to under changes.
Eqn 1 is reduction.Q3. Help please. The answer is B.View attachment 58386
Well you should use this eq. :Cu + 4H+ + 2NO3– → Cu2+ + 2NO2 + 2H2O. Because it is ionic :3When concentrated nitric acid, HNO3, is added to copper turnings, a brown gas is evolved. Use data from the Data Booklet to construct an ionic equation for this reaction.
which equation of copper should i use and why ?
Well, IDK :3 But i guess we can't use Cu+ simply because NO2 and H2O need e- :3why can't we use Cu+ instead of Cu2+ ?
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now