- Messages
- 60
- Reaction score
- 109
- Points
- 43
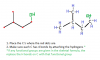
Are you done with the whole syllabus? Actually, I've covered almost whole AS Chemistry but this question is still out of my comprehension. As far as I know, nucleophilic substitution involves halogenalkanes. Since all the halogens are more electronegative than carbon (with the exception of iodine), the electrons are pulled more by the halogens than by the carbon, which leaves the carbon a slightly positive charge while the halogen with a slightly negative charge. Now, the carbon is susceptible to attacks by nucleophiles and attaches with any nucleophile that comes in it's way. Looking at the list of common nucleophiles, NH2 is the one that could be involved here, but the picture presented by the structures show that a hydrogen has been lost, which is positive and could not possibly have acted as a nucleophile!Can you explain how this is nucleophilic substitution View attachment 58706