- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
Yes correct. Because it's 0.5mol/dm3 not 1mol/dm3.Also in this
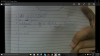
When required to prepare 250 cm3 of 0.5 mol/dm3 solution of a crystal of Mr 50g
First realize that we need only 250 cm3, not 1 dm3
Now in 250 cm3 there will be: 50/4 = 12.5 g of the solid
Shouldn't it be 6.25 g ?
(PS you should not trust this source)