- Messages
- 1,394
- Reaction score
- 12,123
- Points
- 523
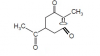
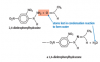
I thought only the CH3 which is near to OH was the only chiral center, because it was the only one which was sp3 hybridizied and had four different groups attached but how is the answer D??View attachment 59774
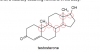
I think these all are the chiral centers for this molecule.