-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 456
- Reaction score
- 280
- Points
- 73
You have to use trial and error.View attachment 60309
plzzzz helppppppppppp
Option A :
The equation: 2Ca + O2 --> 2CaO
Moles of oxygen : 0.3/24 = 1/80
Moles of calcium that will react : 2 × 1/80 = 1/40 (since 2 moles of calcium react with one mole of oxygen.
Moles of metal given in question : 1.15/40 = 23/800
The moles given and the moles that react aren't equal so this is incorrect
Now use this way for all of them. I'll do it for the correct option D
Option D :
The equation: 4Na + O2 --> 2Na2O
Moles of oxygen : 1/80
Moles of sodium that will react : 4 × 1/80 = 1/20
Moles of metal given in question: 1.15/23 = 1/20
They are the same thus D is the ans.
- Messages
- 122
- Reaction score
- 17
- Points
- 28
then why they have given the pressure and temperatureYou have to use trial and error.
Option A :
The equation: 2Ca + O2 --> 2CaO
Moles of oxygen : 0.3/24 = 1/80
Moles of calcium that will react : 2 × 1/80 = 1/40 (since 2 moles of calcium react with one mole of oxygen.
Moles of metal given in question : 1.15/40 = 23/800
The moles given and the moles that react aren't equal so this is incorrect
Now use this way for all of them. I'll do it for the correct option D
Option D :
The equation: 4Na + O2 --> 2Na2O
Moles of oxygen : 1/80
Moles of sodium that will react : 4 × 1/80 = 1/20
Moles of metal given in question: 1.15/23 = 1/20
They are the same thus D is the ans.
- Messages
- 456
- Reaction score
- 280
- Points
- 73
To let u know that the reaction is carried out at room temperature and pressure.then why they have given the pressure and temperature
Because when we find the moles of oxygen we divide by 24. This is only applicable if the reaction is carried out at room temperature. See the first page of data booklet. There are different values of the molar gas constant under different conditions
- Messages
- 456
- Reaction score
- 280
- Points
- 73
- Messages
- 187
- Reaction score
- 976
- Points
- 103
did you see the mark scheme?View attachment 60328
Part (ii). I was wondering is it acceptable if the reagent is some nitrate salt of a metal more reactive than Mg like KNO3 or NaNO3 so a double displacement reaction takes place.
- Messages
- 187
- Reaction score
- 976
- Points
- 103
I don't think it will work because nitric acid is a strong acid which neutralized MgO, on the other hand KNO3 is almost neutral and is moderately soluble in water. I doubt if it'll react at all and of course to be on the safe side, we'd better write what we already came across in our syllabusView attachment 60328
Part (ii). I was wondering is it acceptable if the reagent is some nitrate salt of a metal more reactive than Mg like KNO3 or NaNO3 so a double displacement reaction takes place.
- Messages
- 456
- Reaction score
- 280
- Points
- 73
Alrighty then. ThanksI don't think it will work because nitric acid is a strong acid which neutralized MgO, on the other hand KNO3 is almost neutral and is moderately soluble in water. I doubt if it'll react at all and of course to be on the safe side, we'd better write what we already came across in our syllabus
- Messages
- 456
- Reaction score
- 280
- Points
- 73
- Messages
- 187
- Reaction score
- 976
- Points
- 103
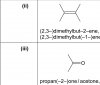
yep, they are alright! The main thing is The alkene should have a double bond in between, which you drew right, and the number of carbons should be same which is also okay...The ketone also has it's functional group all fine and carbon atoms too. It doesn't matter how much we rotate it, or draw it in which way, because it all means the same (remember, as long as we don't count it as another isomer, it's the same molecule)Ik I am asking silly questions but I just want to be on the safe side
The skeletal formula of 2-3 dimethyl but 2 ene and propanone are like this in the msView attachment 60329
This is how I drew themView attachment 60330 they're fine right?
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
- Messages
- 456
- Reaction score
- 280
- Points
- 73
Do I have to use skeletal formula in Paper 4? It seems like all the answers in the markscheme are standardised to use skeletal formula, but I am more comfortable with the displayed formula. I know sometimes they specify but do I have to use if its not specified in the question?
its always better to use whatever is present in the mark scheme if u want to b on a safe sideDo I have to use skeletal formula in Paper 4? It seems like all the answers in the markscheme are standardised to use skeletal formula, but I am more comfortable with the displayed formula. I know sometimes they specify but do I have to use if its not specified in the question?
- Messages
- 25
- Reaction score
- 6
- Points
- 13
http://maxpapers.com/wp-content/uploads/2012/11/9701_w08_qp_4.pdf
please help with Q4 b iii)
please help with Q4 b iii)
- Messages
- 2,738
- Reaction score
- 6,309
- Points
- 523
If it isn't specified in the question, you can use any as long as it is correct.Do I have to use skeletal formula in Paper 4? It seems like all the answers in the markscheme are standardised to use skeletal formula, but I am more comfortable with the displayed formula. I know sometimes they specify but do I have to use if its not specified in the question?
- Messages
- 187
- Reaction score
- 976
- Points
- 103
Where is your K2SO4 formed? I guess you should start writing the way mark scheme advises us cauz it's kinda confusingIs it fine of I write them as 2 separate reactions because that's how it is in the Cambridge book.View attachment 60334
This is how it is in the msView attachment 60335
- Messages
- 2,738
- Reaction score
- 6,309
- Points
- 523
- Messages
- 2,738
- Reaction score
- 6,309
- Points
- 523
- Messages
- 187
- Reaction score
- 976
- Points
- 103
Notice that before hydrolysis, we have total 5 alcohols, one primary alcohol, the one projecting upwards on top left, and the other 4 are all primary, because all of them are bonded to a carbon which, in turn, is bonded to another carbon.Anyone please explain?
View attachment 60340
After hydrolysis, the Cls will be replaced by OH, our primary alcohol remains the same as 1, secondary increase by 1, because another secondary has been added when the left sided chlorine gets replaced by OH. The Cl on the very right, gets replaced by OH as well, but since it is bonded to a carbon atom, which in turn, is bonded to more than 2 carbon atoms, that one becomes a tertiary alcohol. That leaves us with the option C.