- Messages
- 2,738
- Reaction score
- 6,309
- Points
- 523
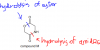
I got it thanks. I had been confusing sth.Notice that before hydrolysis, we have total 5 alcohols, one primary alcohol, the one projecting upwards on top left, and the other 4 are all primary, because all of them are bonded to a carbon which, in turn, is bonded to another carbon.
After hydrolysis, the Cls will be replaced by OH, our primary alcohol remains the same as 1, secondary increase by 1, because another secondary has been added when the left sided chlorine gets replaced by OH. The Cl on the very right, gets replaced by OH as well, but since it is bonded to a carbon atom, which in turn, is bonded to more than 2 carbon atoms, that one becomes a tertiary alcohol. That leaves us with the option C.