- Messages
- 29
- Reaction score
- 8
- Points
- 13
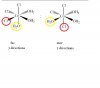
concentration of OH- and H+ ions is too low in aqueous solutions, so this is different from O'levelsFor this question at anode, won't it be OH- that gets oxidized? Why H2O?
never use or show H+ or OH- ions
www.youtube.com/fahadsacademyonline
www.fahadsacademy.com