- Messages
- 665
- Reaction score
- 13,607
- Points
- 503
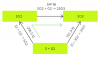
Look here's the thingAns :21/9
The ratio we already have for tertiary : primary is 21 : 1.
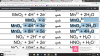
However, as you can see the number of atoms of hydrogen which can be substituted by Cl to make primary halogenoalkane are 9
while in tertiary there is only 1.
Thus, 9 kinds of primary products can be formed but only one kind of tertiary
thus the ratio of products formed tertiary : primary would be 1 : 9
thus the chances of primary product being formed are 9 times greater than tertiary and so products made will be 9 times higher in concentration IF RATE OF REACTION IS NOT CONSIDERED
however, as the reaction RATE is slower, the ratio will be somewhat different
this can be calculated by multiplication
21 x 1 : 1 x 9
thus the ratio would be 21 : 9