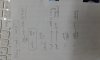Well first write down the volumes of each gas.
1- The initial mixture with H2O + CO2 + O2 has a total volume of 250cm3 but removing H2O gave a volume of 170cm3.
So VOLUME OF H2O = 250cm3 - 170cm3 = 80cm3.
2- The remaining mixture of CO2 + O2 = 170cm3. Removing CO2 with alkali gives 110cm3.
So VOLUME OF CO2 = 170cm3 - 110cm3 = 60cm3.
3- The final mixture only contains O2 which is 110cm3. So out of 2o0cm3, 110cm3 did not react.
so VOLUME of reacted O2 = 200cm3 - 110cm3 = 90cm3.
4- Volume of alcohol = 20cm3.
Now since all are gases (remember water is actually water vapour so its a gas too) , we can take their ratios to find no. of moles.
so
CxHyO + zO2 -> xCO2 + y/2H2O
20 : 90 : 60 : 80
Now in order to find the value of x, we are supposed to take only 1 mole of the alcohol. So we will reduce the ratios down in such a way that the no. of moles of alcohol are 1.
So it'll be like
CxHyO : O2 : CO2 : H2O
1 : 4.5 : 3 : 4
The no. of moles of CO2 r 3 so x = 3.
thanks a looot