- Messages
- 438
- Reaction score
- 3,645
- Points
- 253
WHAT????????????????????????]
its carbon environments so there are 3 different environments in the side chain and 4 different in the benzene ring itself
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
WHAT????????????????????????]
its carbon environments so there are 3 different environments in the side chain and 4 different in the benzene ring itself
too vaguelol i dint quite get this either
my teacher also dint get the concept behind this
but he said that if there are two reactions ans theyre asking for one, the one with phenol should be considered
Ahahahahah read this ^ she explained it wellWHAT????????????????????????
if there were 3 en ligands you could have done optical but in this case its 2 different ligands 2 en's and one other ligand so its cis transBut Ni is bonded to en. Isnt en(NH2CH2CH2NH2) a bidentate ligand?
Amines are soluble in both hot and cold as they ionise by accepting protons.can someone tell about the solubility of amides and amine in HCL? and with cold/hot hcl?
the AgNO3 is for all halides and is usually used to find if halogenoalkane is chloro bromo or iodohttps://papers.gceguide.com/A Levels/Chemistry (9701)/9701_s17_qp_42.pdf for Q7 what is the diff b/w test 1 and 2? aren't the both to test for chloride ions?
hey, what's the diff between the blue and green ones? :/View attachment 63544
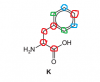
The ones I've circled in red are all having different chemcal environments...these are 5 carbons
The two in blue have the same env. as they are both next to the adjacent C atom in benzene next to the side chain so we will consider this 1 env.
Same goes for both in green...they are BOTH next to the Carbon side chain linked to benzene so both have same env. so v will consider this one env. too
so 1 + 1+ 5 = 7 different env.
Nickel will have cis trans isomers because it has two pairs of different kinds of ligands. Just draw two planar structures.
Also since Ni is not bonded to a bidentate or 4 different ligands, there should be no optical isomerism here.
one is directly bonded to the carbon with the side chainhey, what's the diff between the blue and green ones? :/
there is a difference, in the question for w it says for immediately thus its acyl chloride, for y its very slow so its chloro alkane. Since acyl cholrides are far more reactivethe AgNO3 is for all halides and is usually used to find if halogenoalkane is chloro bromo or iodo
white forms quickly for chlorides, cream white forms slowly with Br and yellow forms with I.
While test 2 is only for chlorides
however, since only chlorine is mentioned in the molecular formula, i dont think there was any point of including two tests :/
you are adding 30cm3 to 20 so 50 in the flask at the end?https://papers.gceguide.com/A Levels/Chemistry (9701)/9701_s17_qp_43.pdf here in 2ci while calculating the conc of OH- at the end why do we use the total volume of 50cm^3 ? wouldn't the volume be used up during titration?
I think since theres excess of Fe ions, they ll first oxidize vanadium metal to V2+, then V2+ to V 3+, then V3+ to VO2+. Since all of them have E less than +0.77There is no equation in the data booklet matching the one given in the mark scheme. How is this answer obtained?
ThanksI think since theres excess of Fe ions, they ll first oxidize vanadium metal to V2+, then V2+ to V 3+, then V3+ to VO2+. Since all of them have E less than +0.77
well no it doesnt matter actuallyView attachment 63545
How do we come to know where to place the Cl and NH3? Cant there be any other arrangement apart from these?
View attachment 63546
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now