-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 665
- Reaction score
- 13,607
- Points
- 503
m looking for em tooAnybody got 9701_m18_qp_42?
but they wont officially be released b4 the 17th :/
- Messages
- 150
- Reaction score
- 222
- Points
- 53
- Messages
- 341
- Reaction score
- 224
- Points
- 53
https://papers.gceguide.com/A Levels/Chemistry (9701)/9701_s17_qp_43.pdf can someone explain 3bii, the coordination and formulae of D AND F?
- Messages
- 438
- Reaction score
- 3,645
- Points
- 253
- Messages
- 438
- Reaction score
- 3,645
- Points
- 253
Kindly post them here as soon as you get them.m looking for em too
but they wont officially be released b4 the 17th :/
- Messages
- 328
- Reaction score
- 84
- Points
- 38
can u pls explain the charges on each?
- Messages
- 28
- Reaction score
- 10
- Points
- 13
It can form only coordination 4 which shows cis trans isomer . For cis chlorine should be 90 degree to each other while for trans both chlorine should be 180 degree to each other . Chorine placed anywhere other then 180 degree will only have 90 degree because all four are on same plane. Not to loose marks just male chlorine next to the other chlorine to show cis isomer.View attachment 63545
How do we come to know where to place the Cl and NH3? Cant there be any other arrangement apart from these?
View attachment 63546
- Messages
- 28
- Reaction score
- 10
- Points
- 13
Im sure you calculated the Mr for i.
1 gram of the sample was taken so you divide the mass by Mr.
You then get the number of moles.
Which will be equal to one of the answers on the table for AgCl. Then you calculate the other values of n simply by looking at the ratios.
(These were just the steps so that you try it yourself) Good Luck.
- Messages
- 438
- Reaction score
- 3,645
- Points
- 253
Charges? I didn't get itcan u pls explain the charges on each?
- Messages
- 438
- Reaction score
- 3,645
- Points
- 253
its simply the number of proton attached to the carbon if its CH3 the relative peak area is 3 if OH the 1 and so on
- Messages
- 438
- Reaction score
- 3,645
- Points
- 253
Simply!!!its simply the number of proton attached to the carbon if its CH3 the relative peak area is 3 if OH the 1 and so on
- Messages
- 665
- Reaction score
- 13,607
- Points
- 503
Also Holmes this is actually the correct explanationAnyone?
well you can only have two isomers
cis and trans
and for the cis isomer, there will be two optical isomers since there is also a bidentate ligand present
THIS IS TRANS
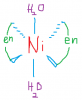
THE ONES BELOW ARE BOTH CIS AND MIRROR IMAGES OF EACH OTHER...THE LINE IN BETWEEN IS THE MIRROR
THIS KIND OF ISOMERISM IS ONLY POSSIBLE WHEN BIDENTATE LIGANDS ARE ALSO PRESENT
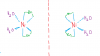
- Messages
- 665
- Reaction score
- 13,607
- Points
- 503
Zaki ali asgharcan u pls explain the charges on each?
i think phenol should ionise in such a high pH aint it?
and i think it is tyr that is P and the phe-tyr and phe are R and S
becuz like phe has a low mass and double the charge since phenol and acid both ionise
and since phe-tyr has double the mass and double the charge than tyr, the movement of both is same in the end
Last edited:
- Messages
- 36
- Reaction score
- 19
- Points
- 8
I dont think this is the case. It would still show optical isomerism.if there were 3 en ligands you could have done optical but in this case its 2 different ligands 2 en's and one other ligand so its cis trans
- Messages
- 438
- Reaction score
- 3,645
- Points
- 253
Great shotAlso Holmes this is actually the correct explanation
well you can only have two isomers
cis and trans
and for the cis isomer, there will be two optical isomers since there is also a bidentate ligand present
THIS IS TRANS
View attachment 63567
THE ONES BELOW ARE BOTH CIS AND MIRROR IMAGES OF EACH OTHER...THE LINE IN BETWEEN IS THE MIRROR
THIS KIND OF ISOMERISM IS ONLY POSSIBLE WHEN BIDENTATE LIGANDS ARE ALSO PRESENT
View attachment 63568
- Messages
- 341
- Reaction score
- 224
- Points
- 53






