-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 27
- Reaction score
- 8
- Points
- 13
It's that time of the year again xD
- Messages
- 500
- Reaction score
- 425
- Points
- 73
You are welcomeThank you so much I get it now!!
- Messages
- 27
- Reaction score
- 8
- Points
- 13
Quick question : When writing an ionic equation from the list in the data booklet , we choose the ones which give us the most Potential difference , right?
I'm not quite sure about it. Sorry...Quick question : When writing an ionic equation from the list in the data booklet , we choose the ones which give us the most Potential difference , right?
- Messages
- 27
- Reaction score
- 8
- Points
- 13
Hot concentrated KmnO4 causes rupture at the double bond. Thus, you break both the double bonds and form ketone and carboxylic acid (according to the arrangement). Also, do remember that CO2 is always produced during rupture.I'm not quite sure about it. Sorry...
I'm not the best when it comes to explaining so if it doesn't clear that up just reply and I'll try sending you a picture.
Edit : Methanioc acid would be a product too but since it's not mentioned we ignore it.
Hot concentrated KmnO4 causes rupture at the double bond. Thus, you break both the double bonds and form ketone and carboxylic acid (according to the arrangement). Also, do remember that CO2 is always produced during rupture.
I'm not the best when it comes to explaining so if it doesn't clear that up just reply and I'll try sending you a picture.
Edit : Methanioc acid would be a product too but since it's not mentioned we ignore it.
Sorry I still don't really get it... Can I see the picture?? Thank you so much!
- Messages
- 27
- Reaction score
- 8
- Points
- 13
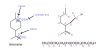
Here you are
I tried but it says that the file size is too big sorry ;-;Sorry I still don't really get it... Can I see the picture?? Thank you so much!
- Messages
- 27
- Reaction score
- 8
- Points
- 13
Also, is there literally anyone who can clear this up?Quick question : When writing an ionic equation from the list in the data booklet , we choose the ones which give us the most Potential difference , right?
- Messages
- 500
- Reaction score
- 425
- Points
- 73
Whenever it says file size too big just take a screenshot of the picture you want then post it.Here you are
I tried but it says that the file size is too big sorry ;-;
- Messages
- 27
- Reaction score
- 8
- Points
- 13
Got it! thank you.Whenever it says file size too big just take a screenshot of the picture you want then post it.
Can you please help me with my question though?
- Messages
- 500
- Reaction score
- 425
- Points
- 73
Sorry I don't know the answer.Got it! thank you.
Can you please help me with my question though?