- Messages
- 561
- Reaction score
- 733
- Points
- 103
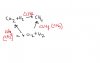
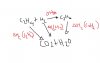
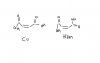
How to calculate enthalpy change of a reaction when enthalpy change of formation or combustions r given??? is it enthalpy change of (products- reactants ) or is it the other way round??? can any1 explain me in details??? littlecloud11