- Messages
- 128
- Reaction score
- 112
- Points
- 53
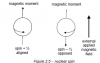
so it was that simple huh. thanks a tonneRead the details given in the question for the compound.
It says it gives white ppt with Br2 aq means it will have a phenol.gives yellow ppt with tri idoform test means there will be COCH3 group present and it is mentioned that it is 1,4 di substituted.so phenol at 1 and COCH3 at 4.got it!