- Messages
- 354
- Reaction score
- 529
- Points
- 103
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Q15. This is a similar to question Q14 of 2006.
Group II nitrates decompose based on the equation below:
2 X(NO3)2 (s) --> 2 XO (s) + 4 NO2 (g) +O2(g)
1.32 g is mass of NO2 and O2 gas, the left over mass 0.68 g is XO.
Mass of XO = 0.68g
Moles of XO = 0.68/ (Mr of X + 16)
Mass of X(NO3)2 = 2 g
Moles of X(NO3)2 = 2/(Mr of X + 62)
since moles of X(NO3)2 = moles of XO
2/(Mr of X + 124) = 0.68/ (Mr of X + 16)
We can solve for Mr of X using the normal maths approach, but it might be faster to do trial and error from the Mr of the four options.
Be : 2/(9 + 124) does not equal to 0.68/ (9 + 16)
Ca: 2/(40 + 124) = 0.68/ (40+ 16)
Therefore, answer is Ca.
Q17. At RTP, 1 mol of gas (assume ideal) occupies 24 000 cm3.
mole of O2 = 300 /24 000 = 0.0125 mol
2Ca + O2 --> 2 CaO
2Mg + O2 --> 2 MgO
4K + O2 --> 2K2O
4Na + O2 --> 2 Na2O
0.0125 mol of O2 will react with:
0.025 mol of Ca (1 g)
0.05 mol of Mg (1.2 g)
0.05 mol of K (1.95 g)
0.05 mol of Na (1.15 g)
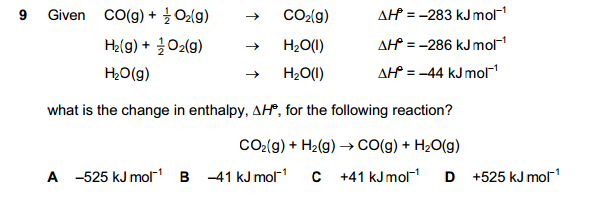
Enthalpy change = Σ(ΔH Products) - Σ(ΔH Reactants)
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w04_qp_1.pdf
Q4
Q19
Q21 i have difficulty on finding a ciral carbon from rings any tips?
Q33 what about 3? more the concentration lower the activation energy?
Q35 waht about2 won't CO2 and O2 be made with it?
Q39 how do we make out that it reacts with sodium
Help please
Does anybody have explanations for chemistry mcqs?
10 juneCould I confirm the date of the chemistry paper?
Hi can someone please tell me why the product is a carboxylic acid not aldehyde while they mentioned distillation takes place !
Q 25 http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w11_qp_13.pdf
But they also said distillation ! So plz tell me under which condition will an aldehyde form?Its clearly written that ' The reaction mixture was then boiled under reflux for one hour' so this means that carboxylic acid is formed not the aldehyde.
But they also said distillation ! So plz tell me under which condition will an aldehyde form?
Honestly, I do see some flaws in the question set.But they also said distillation ! So plz tell me under which condition will an aldehyde form?
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now