Energy is released when these compounds undergo combustion ... combustion is basically reacting with Oxygen And compounds A, C, and D already have oxygen in them and have the same number of carbon atoms as B.. so we can considered them to be partly combusted.. Hence only compound B havent got Oxygen in it .. And so the Ans is Byeah but why don't we consider C-O bonds too?
-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
If a compound has a ring in it,does that mean all bonds will be at angles and there will be no possibility of Cis Trans at any double bond inside the ring?
Metanoia
Trans and cis are possible at the double bonds when the ring has 8 or more carbons.
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
Short note on periodicity chapter? 
Actually one of my friends need it... anyone?
Actually one of my friends need it... anyone?
- Messages
- 6,392
- Reaction score
- 27,001
- Points
- 698
http://www.docbrown.info/page13/page13b.htm
Short note on periodicity chapter?
Actually one of my friends need it... anyone?
- Messages
- 6,392
- Reaction score
- 27,001
- Points
- 698
http://www.s-cool.co.uk/a-level/chemistryShort note on periodicity chapter?
Actually one of my friends need it... anyone?
http://maxpapers.com/wp-content/uploads/2012/11/9701_s13_qp_12.pdf
somone help me out with question 21 guys
somone help me out with question 21 guys
- Messages
- 7
- Reaction score
- 3
- Points
- 13
- Messages
- 7
- Reaction score
- 3
- Points
- 13
The solubility of Group 2 hydroxides increases down the group .. so we can say that Barium hydroxide will be more soluble than calcium hydroxide ... hence when these two solutions are mixed together .. it will be the Calcium hydroxide that will be responsible for the white precipitate due to its less solubility compared to barium hydroxide .. and the filtrate will be containing Barium hydroxide .. And so when CO2 gas is bubbled to this filtrate .. it will react with Barium hydroxide forming Barium carbonate ...
Hope it helps !!!!!!
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
http://maxpapers.com/wp-content/uploads/2012/11/9701_s13_qp_12.pdf
somone help me out with question 21 guys
There are two C=C bonds.
When reacted with H2, it can take in 2 H2 molecules.
When reacted with Br2 it can also take in 2 Br2 molecules.
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
View attachment 54635 the marking scheme says answer is c but how r there 3 geometric isomers???
How many do you have?
Yeah its D thanksif the Ans is D...
- Messages
- 1,318
- Reaction score
- 1,374
- Points
- 173
- Messages
- 1,318
- Reaction score
- 1,374
- Points
- 173
There are 2 double bonds so you can have these combinations:View attachment 54635 the marking scheme says answer is c but how r there 3 geometric isomers???
Cis at the first double bond and Cis at the second
So keep on changing and following the same pattern you can have:
C=cis and T= trans
CC
TT
CT / TC
Since the hex-2,4-diene is optically same (shows the same formula when read backwards and forwards), the CT and TC will count as one (it will just be a mirror image of each other)
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
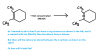
View attachment 54646
Please can someone clarify this doubt?
There will be two oxygen atoms attached to form 2 ketone groups, one for each side.
- Messages
- 1,318
- Reaction score
- 1,374
- Points
- 173
So the ring would open up?There will be two oxygen atoms attached to form 2 ketone groups, one for each side.
- Messages
- 479
- Reaction score
- 1,374
- Points
- 153
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
So the ring would open up?
Thats right.
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123