- Messages
- 3
- Reaction score
- 2
- Points
- 3
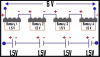
Basically, the electrons produced at the negative terminal moves through the circuit to reach the positive terminal. But when, two or more cells are connected in series, how do the cells contribute their electrons into the circuit? for example, the 2nd cell sandwiched between the 1st and 3rd cell, how does its electrons reach the circuit wire?