Q.26: You have to consider that there's an intermediate so there's two humps. The front hump is always higher than the second hump. I'm not sure why but it's just a basic rule, I think. XD
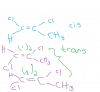
Q.27: Hydrolysis means substituting Br with OH group. So if all the Br are substituted, they would give you the same diol, wouldn't they? It's a nucleophilic substition reaction. And only the second organic compound can form H bond due to presence of OH group.
Q.33: CAtalyst does not increase KE of molecules. They lower the activation e so that molecules with lower KE can react. Catalyst also increases the rate of reaction whether it's backward or forward. It doesn't affect enthalpy change, same amount of energy released or used by reaction whether catalyst is there or not. It just speeds up the reaction.
Q.35: For the first: CaO + SO2 = CaSO3 thats correct
second: SO2+O2 = 2SO3 due to excess air then CaO + SO3 = CaSO4
third: CaO + CO = CaCO3 (lazy to balance) this is not a likely reaction as CO is neutral (but CO2 is acidic!) My lecturer helped with this question
Bro for question 35, SO2 need certain special conditions to covert to SO3 not only excess of air. So option 1 and 2 are both correct I mean B and D. Can you please explain?