cause they say that each mole produces H+ ions in the questionfishcook explains it in a good way,
-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Physics, Chemistry and Biology: Post your doubts here!
- Thread starter semsemhosam
- Start date
- Messages
- 214
- Reaction score
- 138
- Points
- 53
hmm ok actually its for 2000 so forge titi think you find it out by titration, using an indicator and comparing the volumes of acid used to neutralize lets say 25cm3 of NaOH
the one that uses less acid is H2Y so the one that uses more is HY... Im not sure though.
- Messages
- 214
- Reaction score
- 138
- Points
- 53
i dun have link! xtreem papers dsnt havesorry, link plz?
- Messages
- 1,162
- Reaction score
- 6,306
- Points
- 523
5n i think fishcook gave d ryt 1.i dun have link! xtreem papers dsnt have
- Messages
- 214
- Reaction score
- 138
- Points
- 53
plzzz explain how did u doMg + 2H+ > Mg+2 + H2
- Messages
- 1,162
- Reaction score
- 6,306
- Points
- 523
mg is in metal state, so no charge. h is in acid so +(coz H is always +ve), in H2SO4, so 2 hydrogen atoms, so 2H+ ionsplzzz explain how did u do
im a bad xplainer, so sorry if u didnt get it
- Messages
- 223
- Reaction score
- 80
- Points
- 38
really, no, y do dat?
lyk acid donates H+ ions, rite? plz reply asap, n thx
if you think about it...if you are going to get rid of both OH from the alcohol why put it in the first place ??
In condensatio polymarization, polyeters, you have dicarboxylic acid and dialcohol ... so when you come to remove the said elemants to have a polymer, you remove the OH from the acid, (Both) and H from the alcohol (both).
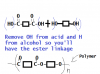
sorry bad drawing but the best i can to show you!!
- Messages
- 859
- Reaction score
- 360
- Points
- 73
Alright..d- the formula of an acid is either of the type HY or H2Y.How could you find out which one is correct by using Aq.solutions of the acid and of sodium hydroxide. Assume that both solutions have the same concentration, 0.1 mol/dm3
please help!
HY-monoprotic(HCL, HNO3)
H2Y-diprotic (H2CO3,H2SO4)
By Titration
NaOH +HCl -->NaCl +H2O
Ratio :1:1
2NaOH +H2SO4-->NaSO4 +H2O
Ratio :2:1
Prepare the same conc of NaOH as the unknown acid
pipette out 25cm3 of unknown acid and add a few drops of suitable indicator
Fill burette with NaOH solution till zero Mark and run the burette dropwise until indicator just changes colour
record the volume of NaOH used
If the volume ratio is 1:1 then the acid is HY type else H2Y type
- Messages
- 136
- Reaction score
- 27
- Points
- 38
plzzz explain how did u do
ok
the normal equation is:
Mg + H2SO4 -> MgSO4 + H2
anything (aq) must be written in ion format, anything else SAME
Mg + H+ + SO4-2 -> Mg+2 + SO4- + H2
delete ions that are same in the reactants and the products sides (in this case only sulphate, note that Mg is solid and Mg+2 is ion so dont delete, and H2 is gas and H+ is ion so dont delete)
Mg + H+ -> Mg+2 + H2
Balance
Mg + 2H+ -> Mg+2 + H2
- Messages
- 1,162
- Reaction score
- 6,306
- Points
- 523
oyee, 2NaOH +H2SO4-->Na2SO4 + 2H2O, i thinkAlright..
HY-monoprotic(HCL, HNO3)
H2Y-diprotic (H2CO3,H2SO4)
By Titration
NaOH +HCl -->NaCl +H2O
Ratio :1:1
2NaOH +H2SO4-->NaSO4 +H2O
Ratio :2:1
Prepare the same conc of NaOH as the unknown acid
pipette out 25cm3 of unknown acid and add a few drops of suitable indicator
Fill burette with NaOH solution till zero Mark and run the burette dropwise until indicator just changes colour
record the volume of NaOH used
If the volume ratio is 1:1 then the acid is HY type else H2Y type
im getting all wrong notions?
- Messages
- 223
- Reaction score
- 80
- Points
- 38
what i mean that there is should be double bonded C
C=C
(generally in esters, i hope you got what i mean)
no the double bond is C=O because C=C is alkenes
- Messages
- 136
- Reaction score
- 27
- Points
- 38
no the double bond is C=O because C=C is alkenes
i knew about just was lazy to correct (the question was talking about acid and not ester
- Messages
- 859
- Reaction score
- 360
- Points
- 73
yea sorry forgot to balanceoyee, 2NaOH +H2SO4-->Na2SO4 + 2H2O, i think
im getting all wrong notions?
- Messages
- 859
- Reaction score
- 360
- Points
- 73
61?can anyone help me with may june 2009 paper 61 question 5 b?
- Messages
- 214
- Reaction score
- 138
- Points
- 53
no no thnx i got it but y in the product thingy H has no charge Mg + 2H+ > Mg+2 + H2mg is in metal state, so no charge. h is in acid so +(coz H is always +ve), in H2SO4, so 2 hydrogen atoms, so 2H+ ions
im a bad xplainer, so sorry if u didnt get it
yes!
- Messages
- 859
- Reaction score
- 360
- Points
- 73
subject?yes!
- Messages
- 214
- Reaction score
- 138
- Points
- 53
i got u like i knw how to do thisok
the normal equation is:
Mg + H2SO4 -> MgSO4 + H2
anything (aq) must be written in ion format, anything else SAME
Mg + H+ + SO4-2 -> Mg+2 + SO4- + H2
delete ions that are same in the reactants and the products sides (in this case only sulphate, note that Mg is solid and Mg+2 is ion so dont delete, and H2 is gas and H+ is ion so dont delete)
Mg + H+ -> Mg+2 + H2
Balance
Mg + 2H+ -> Mg+2 + H2
but when we have to make silver chloride we'll do this?
normal eq- AgNO3 + NaCl ------> AgCl + NaNO
NOW how to make this an ionic eq?