- Messages
- 859
- Reaction score
- 360
- Points
- 73
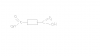
Look in the acid hydrogen is H+ ,what does this mean? it has lost electron and it became an ionno no thnx i got it but y in the product thingy H has no charge Mg + 2H+ > Mg+2 + H2
Now in this reaction Mg is more reactive n it wants to take hydrogen's place , so it becomes very generous and donates it's electron to hydrogen
so hydrogen gains those electron back ,Therefore it's 2H+ to H2