Mind I ask is do they mention this in the syllabus? :3Please what are cladistics...please give examples
-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Physics, Chemistry and Biology: Post your doubts here!
- Thread starter semsemhosam
- Start date
- Messages
- 38
- Reaction score
- 2
- Points
- 8
It is mentionedMind I ask is do they mention this in the syllabus? :3
Sorry cant help you.It is mentioned
Syllabus 2016-2018 ?
- Messages
- 38
- Reaction score
- 2
- Points
- 8
2015Sorry cant help you.
Syllabus 2016-2018 ?
- Messages
- 178
- Reaction score
- 97
- Points
- 38
Thank's Bruh...Well most of these questions have similar answers :
(f) For the first 2.. u just have to read the value from the graph.. i suppose thats pretty simple
(g) For the chemical reaction: If temperature increases.. it'll always be exothermic.. and if temperature decreases then endothermic..other than that if a precipitate is formed they'll mention it in the question.. so its a precipitation reaction....and if its just acid base given and no reference to temperature.. than u can write neutralisation reaction
(h) always remember that volume is inversely proportional to concentration... so write now they have doubled the volume so the concentration will be halved... consequently the temperature change will be less/ halved.
(i) the answer to this will always be the initial temperature u recorded... the first reading from the table ...ALWAYS
these type of questions will have similar answers to the above.. except the numbers will be changed
- Messages
- 2
- Reaction score
- 0
- Points
- 1
can anyone help me out with salt analysis in chemistry (paper 6)
any resources or guidance would be helpful. THANKS.
any resources or guidance would be helpful. THANKS.
- Messages
- 456
- Reaction score
- 280
- Points
- 73
U mean as in to identify the type of salt present?can anyone help me out with salt analysis in chemistry (paper 6)
any resources or guidance would be helpful. THANKS.
- Messages
- 2
- Reaction score
- 0
- Points
- 1
Yes.U mean as in to identify the type of salt present?
- Messages
- 456
- Reaction score
- 280
- Points
- 73
- Messages
- 456
- Reaction score
- 280
- Points
- 73
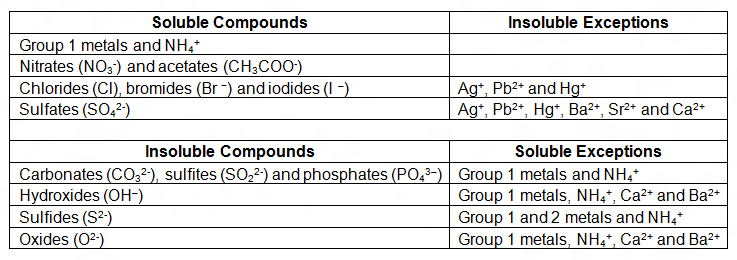
Just learn these rules
- Messages
- 456
- Reaction score
- 280
- Points
- 73
Why hasn't the timer for M/J session 2015 started yet..!? 




- Messages
- 195
- Reaction score
- 49
- Points
- 38
salam every1
well i need help with this question of p6
first, is the second graph correct consedering part (e)
and second question is that in part (d) I wrote that all the acid was used up but answer was all zinc used up as acid was in excess so i want to know that how would we knw weather zinc is in excess or acid
well i need help with this question of p6
first, is the second graph correct consedering part (e)
and second question is that in part (d) I wrote that all the acid was used up but answer was all zinc used up as acid was in excess so i want to know that how would we knw weather zinc is in excess or acid
Attachments
- Messages
- 456
- Reaction score
- 280
- Points
- 73
Lol.. How will the acid get used up... since they are constantly adding more and more HCL until no more reaction happens and the SAME mass of zinc was being used so obviously the acid will be in excess and zinc will be reacted (used up) completelysalam every1
well i need help with this question of p6
first, is the second graph correct consedering part (e)
and second question is that in part (d) I wrote that all the acid was used up but answer was all zinc used up as acid was in excess so i want to know that how would we knw weather zinc is in excess or acid
No the graph u've drawn is incorrect...zinc granules have bigger surface area than powder...so i hope u know that if surface area increases reaction rate decreases thus the the graph will be less steep hence BELOW the original graph... since the same mass of zinc (which means same number of moles) is being used same volume of gas produced...
- Messages
- 195
- Reaction score
- 49
- Points
- 38
Lol.. How will the acid get used up... since they are constantly adding more and more HCL until no more reaction happens and the SAME mass of zinc was being used so obviously the acid will be in excess and zinc will be reacted (used up) completely
No the graph u've drawn is incorrect...zinc granules have bigger surface area than powder...so i hope u know that if surface area increases reaction rate decreases thus the the graph will be less steep hence BELOW the original graph... since the same mass of zinc (which means same number of moles) is being used same volume of gas produced...
Thank u very much for ur help...
- Messages
- 195
- Reaction score
- 49
- Points
- 38
Btw as far as i knw when S.A increases, the rate of reaction increases? No?Lol.. How will the acid get used up... since they are constantly adding more and more HCL until no more reaction happens and the SAME mass of zinc was being used so obviously the acid will be in excess and zinc will be reacted (used up) completely
No the graph u've drawn is incorrect...zinc granules have bigger surface area than powder...so i hope u know that if surface area increases reaction rate decreases thus the the graph will be less steep hence BELOW the original graph... since the same mass of zinc (which means same number of moles) is being used same volume of gas produced...
- Messages
- 154
- Reaction score
- 54
- Points
- 38
Btw as far as i knw when S.A increases, the rate of reaction increases? No?
Yes, that's true.
But granules have smaller surface area, since they have exposure to less reacting particles.
When you make it powder, then surface area increases with more exposure.
- Messages
- 195
- Reaction score
- 49
- Points
- 38
Yes, that's true.
But granules have smaller surface area, since they have exposure to less reacting particles.
When you make it powder, then surface area increases with more exposure.
thanks. ....
- Messages
- 7
- Reaction score
- -1
- Points
- 11
plz if anyone have idea about the phy 0625 (2015) exam plz send it to me my exam after few weeks. THANKS
[email protected]
[email protected]
- Messages
- 195
- Reaction score
- 49
- Points
- 38
When is ur exam? And which variant?plz if anyone have idea about the phy 0625 (2015) exam plz send it to me my exam after few weeks. THANKS
[email protected]