- Messages
- 108
- Reaction score
- 48
- Points
- 38
Thanks but this method can be used on any shape r8Draw it like this:
View attachment 52857
And then just write that X(1) + X(2) / 2 should be equal to 89 cm
Hope u didn't mind me answering the question!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Thanks but this method can be used on any shape r8Draw it like this:
View attachment 52857
And then just write that X(1) + X(2) / 2 should be equal to 89 cm
Hope u didn't mind me answering the question!
Yes, just draw it according to the shape.............. WelcomeThanks but this method can be used on any shape r8
Thanks a lot My NameTHE REACTIVITY SERIES
Please- Potassium
Send- Sodium
Chales- Calcium
McClean- Magnesium
A- Aluminium
Coloured- Carbon
Zebra- Zinc
If- Iron
Nicky- Nickel
The- Tin
Lame- Lead
Horse- Hydrogen
Can't- Copper
Much- Mercury
Some- Silver
Grass- Gold
Properly- Platinum
Hope that helps you guys.
zahra azam Here it is ^
You're welcome!Thanks a lot My Name
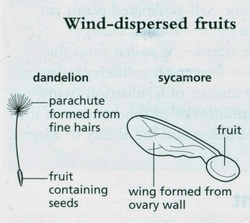
CAN ANY ONE HELP ME IN MAYJUNE 2011 QUESTION 2 BIOLOGY PAPER 6 ITS A DANDELION FRIUT HOW TO LABEL IT
Thank you very much!!!Since they say that the temperature remains constant, P1V1 = P2V2 is used...........
Now they have given us the pressure and volume of the helium in the cylinder and the pressure that normally is in the balloon
What we have to calculate is the volume that will be present in the balloon at that pressure because 0.0030 (m3) is clearly not the volume of the gas in the balloon that has that pressure
So we apply the PV = constant formula and calculate the volume of the gas in the balloon which we get as 0.15 (m3)
Next, we have to subtract this from the original volume because when we r working out the number of INFLATED balloons, this balloon doesn't come in the category
Then we divide the result by the volume that is normally in an inflated balloon and u get the answer
Hope that helps and do tell me if I am wrong
Remember me in ur prayers!!!
Wait a minute i still dont understand that minus part why do we minus the volume of the air in cylinder from the volume that we calculated???Since they say that the temperature remains constant, P1V1 = P2V2 is used...........
Now they have given us the pressure and volume of the helium in the cylinder and the pressure that normally is in the balloon
What we have to calculate is the volume that will be present in the balloon at that pressure because 0.0030 (m3) is clearly not the volume of the gas in the balloon that has that pressure
So we apply the PV = constant formula and calculate the volume of the gas in the balloon which we get as 0.15 (m3)
Next, we have to subtract this from the original volume because when we r working out the number of INFLATED balloons, this balloon doesn't come in the category
Then we divide the result by the volume that is normally in an inflated balloon and u get the answer
Hope that helps and do tell me if I am wrong
Remember me in ur prayers!!!
YUP ITS CORRECTCan someone tell that is my answr for last part correct?
The mass of one mole of an alcohol is 116....what is the molecular formula of the alcohol...please show me steps and thanks in advanceThe minimum minimum margin for all the three subjects is usually about 30 marks (like losing 30 marks)
I'd say DO solve past paper it helps you to know what parts you need to focus on and also the way the questions can be asked.PEOPLE CAN I JUST UNDERSTAND ICT THERY CHAP AND DONT DO PASTPAPER OR IF I JUST DO 1 OR 2 PASTPAPER WOULD I GET GOOD MARKS
1 mol alcohol = 116/molecular mass of alcoholThe mass of one mole of an alcohol is 116....what is the molecular formula of the alcohol...please show me steps and thanks in advance
Okay (lol) we subtract it coz that volume has been used that is it is no longer in the cylinderWait a minute i still dont understand that minus part why do we minus the volume of the air in cylinder from the volume that we calculated???
Welcome!!! I am glad it helped!!!Thank you very much!!!
it was very helpful
I agree with My Name, you should solve at least like two years so that u get a clear idea of the type of questions asked and how to answer them!PEOPLE CAN I JUST UNDERSTAND ICT THERY CHAP AND DONT DO PASTPAPER OR IF I JUST DO 1 OR 2 PASTPAPER WOULD I GET GOOD MARKS
Exactly and also try variants if you have the time.I agree with My Name, you should solve at least like two years so that u get a clear idea of the type of questions asked and how to answer them!
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now