good sources you have.some question on capacitor is coming
-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Physics: Post your doubts here!
- Thread starter XPFMember
- Start date
can i actually write "(13 +- 2) x10^2 " or does it have to be 13x10^2 and then 2x10^2 since theres a +_ sign between?not that the above is percentage uncertainties.
actual values should be given to a SINGLE sf (without taking into account the zeros.)
the above could also be written as (13 +- 2) x10^2 - hence 1sf
See how the final answer is given at the link there.
yeah but I mean, they are not on the same like dots?yes you can use (13 +- 2) x10^2.
actually, you should be using it this way
Like the answer format is like ............ +_ ................
Can I put a big bracket around it then x10^-2?
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
Ready for paper 22 guys? <3
- Messages
- 4
- Reaction score
- 3
- Points
- 3
Precision is how closely repeated measurements of the same quantity agree. It can be thought of as the number of decimal points the quantity is measured to, and is regardless of whether the measurement is correct or notCan someone explain precision and accuracy please??\
also long range order and short range order for materials
Accuracy is how close the measurements are to the true value.
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
Long range order is like solids, the particles are aligned for large distances.Can someone explain precision and accuracy please??\
also long range order and short range order for materials
Short range order is like liquids. They have some order for a small number of particles, but over larger numbers they aren't perfectly aligned.
Yeah I don't get why precision is linked to decimal places? if the values we get for gravity are 9.80 and 9.82, these are precise, what do decimal places have to do with this?Precision is how closely repeated measurements of the same quantity agree. It can be thought of as the number of decimal points the quantity is measured to, and is regardless of whether the measurement is correct or not
Accuracy is how close the measurements are to the true value.
I mean long range and short range for crystalline, amorphous and polymersLong range order is like solids, the particles are aligned for large distances.
Short range order is like liquids. They have some order for a small number of particles, but over larger numbers they aren't perfectly aligned.
- Messages
- 1,318
- Reaction score
- 1,374
- Points
- 173
- Messages
- 456
- Reaction score
- 280
- Points
- 73
- Messages
- 27
- Reaction score
- 5
- Points
- 13
http://papers.xtremepapers.com/CIE/...nd AS Level/Physics (9702)/9702_w09_qp_22.pdf
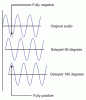
Q5 part b. idk why the mark scheme says that the phase difference is 180.
Q5 part b. idk why the mark scheme says that the phase difference is 180.
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
Hope this pic helps u to understand this concept.http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Physics (9702)/9702_w09_qp_22.pdf
Q5 part b. idk why the mark scheme says that the phase difference is 180.
Good luck.
Attachments
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
So there are two parts to this race:Please help me solve Q9!View attachment 57255
1. Increasing speed from 0 to 10
2. Going at constant speed of 10
We can find time taken for both, and add them up.
1. u=0, v=10, a=2.5, s=?, t=?
First let's find distance covered:
v^2 = u^2 + 2as
100 = 0 + 2(2.5)s
s=20m
So he covered 20m.
Also time taken:
v=u+at
10=0+2.5*t
t=4s
So he took 4s
2. Since he covered 20m in first section, he has to complete 80m more. (100-20)
time = distance / speed = 80/10=8s
Total time = 4 + 8 = 12s
- Messages
- 456
- Reaction score
- 280
- Points
- 73
Oh thank you so much!So there are two parts to this race:
1. Increasing speed from 0 to 10
2. Going at constant speed of 10
We can find time taken for both, and add them up.
1. u=0, v=10, a=2.5, s=?, t=?
First let's find distance covered:
v^2 = u^2 + 2as
100 = 0 + 2(2.5)s
s=20m
So he covered 20m.
Also time taken:
v=u+at
10=0+2.5*t
t=4s
So he took 4s
2. Since he covered 20m in first section, he has to complete 80m more. (100-20)
time = distance / speed = 80/10=8s
Total time = 4 + 8 = 12s
Would appreciate it if u could help in this one as well ( Q6 )
Attachments
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
P = I^2 * ROh thank you so much!
Would appreciate it if u could help in this one as well ( Q6 )
So percentage uncertainty in P = 2 * percentage uncertainty in I + percentage uncertainty in R
Percentage uncertainty in I = 0.05/2.5 * 100% = 2%
Percentage uncertainty in R = 2% (given in question)
Percentage uncertainty in P = 2*2% + 2% = 6%
- Messages
- 27
- Reaction score
- 5
- Points
- 13
thank youHope this pic helps u to understand this concept.
Good luck.
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
KE because the alpha would be moving (KE = 1/2 mv^2 - if something is moving, it has KE)
charge of electron = 1.6x10^-19
1 M (mega) = 10^6
1MeV = (10^6) x (1.6x10^-19) = 1.6x10^-13
5.3MeV = 5.3 x 1.6x10^-13
http://papers.xtremepapers.com/CIE/...and AS Level/Physics (9702)/9702_w07_qp_2.pdf
4c
why don't we use 2x1.9x10^3 for the force since it acts from both directions? shouldn't we double it? Also whats minimum area and whats maximum area? what exactly is happening here because i dont get it at all. would also like explanation for 4d
Can someone give me an ideal answer to give in
http://papers.xtremepapers.com/CIE/...nd AS Level/Physics (9702)/9702_s15_qp_22.pdf
4bi ( kinetic model) and here http://papers.xtremepapers.com/CIE/...nd AS Level/Physics (9702)/9702_s13_qp_21.pdf 4b
(same question) please urgent my exam is tomorrow
http://papers.xtremepapers.com/CIE/...nd AS Level/Physics (9702)/9702_s15_qp_22.pdf
4bi ( kinetic model) and here http://papers.xtremepapers.com/CIE/...nd AS Level/Physics (9702)/9702_s13_qp_21.pdf 4b
(same question) please urgent my exam is tomorrow
http://papers.xtremepapers.com/CIE/...nd AS Level/Physics (9702)/9702_s14_ms_22.pdf
why cant i say interfere in 7a
why cant i say interfere in 7a