- Messages
- 500
- Reaction score
- 425
- Points
- 73
Thank you so much
Are you a graduate?
You are welcome.
No I am currently in As have my paper tomorrow.
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Thank you so much
Are you a graduate?
OMG ._.You are welcome.
No I am currently in As have my paper tomorrow.
View attachment 63988
Sorry for asking too many questions
Can you explain the above one
What is the reason behind slower reaction
Ans is D
Doesn't it form 120 as well?Carbon should be labelled.
And for carbon atoms to be in the same plane the angle between them should be 120 degrees.
View attachment 63981
i get it thanks a lot!This is secondary alcohol but the point is, the sec alcohol must be like this ... "CH(OH)CH3" [Methyl group attached to the same carbon which is attached with OH group]
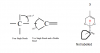
Guys, teach me how to know the type of hybridization for each of the options. I really don't know how( HelpppView attachment 63994
Oct-1-ene, CH3(CH2)5CH=CH2, can be thermally cracked. Which combination of compounds W, X, Y and Z can be obtained by thermally cracking oct-1-ene?
W CH2=CH2
X CH3CH=CH2
Y CH3CH2CH3
Z CH2=CHCH=CH2
A W, X, Y and Z
B W, X and Y only
C W, X and Z only
D W and X only
Doesn't it form 120 as well?View attachment 63995
Thanks a lot
yes the ans is CPlease First tell me is the answer to this question option C .
sp-triple bond
sp2 - double bond
sp3 - single bond
Thanksyes the ans is C
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now