- Messages
- 35
- Reaction score
- 18
- Points
- 18
h
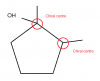
how do you know this?Please First tell me is the answer to this question option C .
sp-triple bond
sp2 - double bond
sp3 - single bond