- Messages
- 8,392
- Reaction score
- 9,462
- Points
- 573
No problem.got it thanks salman nad mayedah
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
No problem.got it thanks salman nad mayedah
we dont even need OCTANE in this question yar.I don't think that's possible becuase, that would be the mass of Octane and to calculate the mass of Carbon dioxide, shouldn't we use the mole ratio?
but H2O is also formed as a product right? The amount should therefore be shared between the two?salman we only need co2 emitted it says for 1km 108g co2 is formed so for 100 km ofcourse 10800g co2 will be formed or emitted
that is something that isnt mentioned in the question we cant assume things unless its stated its only stated for 1 km this formed so for 100 x forms....use cross multiplication all other aspects i dont think we need to consider and I GUESS that 1km for which it says 108 g maybe its the final carbon mass after sharing for 1 km then what us nazr se dekho question kobut H2O is also formed as a product right? The amount should therefore be shared between the two?
Ahan but bro see the equationthat is something that isnt mentioned in the question we cant assume things unless its stated its only stated for 1 km this formed so for 100 x forms....use cross multiplication all other aspects i dont think we need to consider and I GUESS that 1km for which it says 108 g maybe its the final carbon mass after sharing for 1 km then what us nazr se dekho question ko
SORRY! I mis-understood the question! Sorry and Thanks :*then the question says for 1 km 108g co2 is formed who knows that this 108 is final co2 formed along with h20??
JazakAllah for sharingOk i dont mean to do spam or anything but please go to the following thread and make dua for all of us :
http://www.xtremepapers.com/community/threads/dua-for-all-candidates.14272/
Dont forget to share and Remember every share makes a difference
Stephen Pople is a nice and detailed bookpleeeease someone help .... which book is best for physics last few chapters... practical electricity to radioactivity! really worried
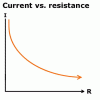
What is the relationship of VOLTAGE with RESISTANCE ?? and how would a RESISTANCE/CURRENT graph look like ??
not true... o2 is liberated if the other negative ion is not concentratedAll those who were worried why it was Cl- ion which was being discharged...i got this from the Xtreme Revision...
"Oxygen from OH- from water is always discharged at the anode except in one case, this is if the other negative ion is a halide"
What is the relationship of VOLTAGE with RESISTANCE ?? and how would a RESISTANCE/CURRENT graph look like ??
According to Ohm's law i.e. E=IR. When we increase resistance, keeping current constant, voltage will increase as in the above formula since E and R are directly proportional.voltage and resistance are inversely proportional if you keep the current constant as u can see from V=IR
and as R and I are inversely propotional then you can see that the graph would be like thisView attachment 8540
Yes its right now !Please check again
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now