No i think oxidant is the substance that get oxidized......r u sure about ur statement?yes of course it is
-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Biology; Chemistry; Physics: Post your doubts here!
- Thread starter scouserlfc
- Start date
- Messages
- 375
- Reaction score
- 226
- Points
- 53
no oxidants are oxidising agents !!! Seriously
- Messages
- 1,824
- Reaction score
- 949
- Points
- 123
no oxidants are oxidising agents !!! Seriously
If Oxidants are Compounds which Oxidize Other compounds then yes.
I think its decreased ! Correct me if wrong
Thankyou bothIf the volume is increased, concentration must decrease and therefore rate will decrease.
I guess
- Messages
- 3,809
- Reaction score
- 3,115
- Points
- 273
volume and concentration are inversely proportional!!Thankyou both
- Messages
- 1,035
- Reaction score
- 539
- Points
- 123
can someone please post all the names of indicators and their colour changes ??
- Messages
- 389
- Reaction score
- 147
- Points
- 53
- Messages
- 450
- Reaction score
- 1,268
- Points
- 143
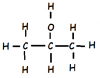
i think it can. dunt know...STATE WHY THIS CAN NOT GIVE A CARBOXYLIC ACID IN PRODUCT WHILE OTHER ISOMER OF IT CAN GIVE IT ..!!?? AND WHAT ITS NAME MEANS PROPANOL-2-OL ..!!!
View attachment 10441
the name means that the OH group is linked to the second carbon
- Messages
- 690
- Reaction score
- 321
- Points
- 73
The OH should be on the side of the structure i guess.
- Messages
- 1,035
- Reaction score
- 539
- Points
- 123
alcohol's functional group is OH not O-H , there is no bond between O and H , thats whats wrongSTATE WHY THIS CAN NOT GIVE A CARBOXYLIC ACID IN PRODUCT WHILE OTHER ISOMER OF IT CAN GIVE IT ..!!?? AND WHAT ITS NAME MEANS PROPANOL-2-OL ..!!!
View attachment 10441
- Messages
- 389
- Reaction score
- 147
- Points
- 53
THNX FOR THE NAMEi think it can. dunt know...
the name means that the OH group is linked to the second carbon
BUT IN PASTPAER ATP A QUESTION CAME
Q) a student found that different alcohol,altough having the same formula did not give a carboxylic acid as the product,suggest the name and structure of this alcohol
name.....................................
structure __________________________
so i posted its answer asking its reasons ..!! pls confirm me soon
- Messages
- 389
- Reaction score
- 147
- Points
- 53
alcohol's functional group is OH not O-H , there is no bond between O and H , thats whats wrong
absolutely not ..the question said that its an ISOMER so how it can be wrong OH or O-H means same
- Messages
- 375
- Reaction score
- 226
- Points
- 53
STATE WHY THIS CAN NOT GIVE A CARBOXYLIC ACID IN PRODUCT WHILE OTHER ISOMER OF IT CAN GIVE IT ..!!?? AND WHAT ITS NAME MEANS PROPANOL-2-OL ..!!!
View attachment 10441
Alcohols with -CH2-OH can only be converted to carboxylic acids !!
- Messages
- 375
- Reaction score
- 226
- Points
- 53
Year please !THNX FOR THE NAME
BUT IN PASTPAER ATP A QUESTION CAME
Q) a student found that different alcohol,altough having the same formula did not give a carboxylic acid as the product,suggest the name and structure of this alcohol
name.....................................
structure __________________________
so i posted its answer asking its reasons ..!! pls confirm me soon
- Messages
- 389
- Reaction score
- 147
- Points
- 53
The OH should be on the side of the structure i guess.
dats what the difference in isomers but why cant it can produce carboxylic ????????????????/ thats the real confusion !!!!!!!!!!
- Messages
- 690
- Reaction score
- 321
- Points
- 73
That's the same thingalcohol's functional group is OH not O-H , there is no bond between O and H , thats whats wrong
- Messages
- 389
- Reaction score
- 147
- Points
- 53
Year please !
SURE =) W-2006-QUESTION 3 PART D
- Messages
- 690
- Reaction score
- 321
- Points
- 73
That's my answer lol, in this isomer, OH is in the middle, so maybe its more stable like this. Besides, the carboxyl group is always on the end of the structure so it can not be oxidised! MY GUESS.dats what the difference in isomers but why cant it can produce carboxylic ????????????????/ thats the real confusion !!!!!!!!!!
- Messages
- 389
- Reaction score
- 147
- Points
- 53
That's my answer lol, in this isomer, OH is in the middle, so maybe its more stable like this. Besides, the carboxyl group is always on the end of the structure so it can not be oxidised! MY GUESS.
may be ..!! you are right !! seems valid reasonn!!
- Messages
- 16
- Reaction score
- 2
- Points
- 3
THNX FOR THE NAME
BUT IN PASTPAER ATP A QUESTION CAME
Q) a student found that different alcohol,altough having the same formula did not give a carboxylic acid as the product,suggest the name and structure of this alcohol
name.....................................
structure __________________________
so i posted its answer asking its reasons ..!! pls confirm me soon
isomer will change the chemical properties so i guess thts why it wudnt react