- Messages
- 3,809
- Reaction score
- 3,115
- Points
- 273
I'll conform this from Imran Merchant.Best Chemistry teacher.
well he is right...
its considered to be concentrated..
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
I'll conform this from Imran Merchant.Best Chemistry teacher.
well he is right...
its considered to be concentrated..
yes, because, in our syllabus, only these electrolytes are mentioned:
(e) apply the idea of selective discharge (linked to the reactivity series for cations, see 9.2) to deduce the
electrolysis of concentrated aqueous sodium chloride, aqueous copper(II ) sulfate and dilute sulfuric acid
using inert electrodes
and moreover, even in our books, there is no mention of production of oxygen while electrolysing Aqueous Sodium chloride, though it is mentioned that chlorine is preferentially discharged at anode and hydrogen at cathode and the remaining solution contains sodium and hydroxide ions, so it becomes an alkali. (NaOH)
http://www.xtremepapers.com/papers/CIE/Cambridge International O Level/Physics (5054)/5054_w09_qp_2.pdf
I need help in Question 7 part a.
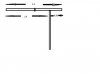
They have written to find the distance from the centre of mass from the wall so the perpendicular distance to be found out is to the other side !I got answer 1 : reason center of gravity is in center therefore at a distance of 1.5 m now check its distance from stand i.e 1.5-0.5 = [ 1] View attachment 8426
please conform it-not sure

The involvement of water accounts for the fact that rusting occurs much more rapidly in moist conditions as compared to a dry environment such as a desert. Many other factors affect the rate of corrosion. For example the presence of salt greatly enhances the rusting of metals. This is due to the fact that the dissolved salt increases the conductivity of the aqueous solution formed at the surface of the metal and enhances the rate of electrochemical corrosion. This is one reason why iron or steel tend to corrode much more quickly when exposed to salt (such as that used to melt snow or ice on roads) or moist salty air near the ocean.can anyone plz explain tht wat effect does salt has on rate of rusting of iron? n y??
i was doing moles questions and in one question my answer was o.oo10035 moles but in marking scheme is was 0.001 moles .. can anyone please tell me a standardized method of writing moles .. like till how many decimal places or significant figures ?
They have written to find the distance from the centre of mass from the wall so the perpendicular distance to be found out is to the other side !
Ur answer is right but we will divide 3 by 2 to find centre of mass which will be 1.5 then subtract 1.5 from 2.5 to find the distance of wall from the centre of slab
When current increases does voltage increase?....when resistance increase does voltage increase?...when resistance increase does current decrease?....when power increase what happens to the voltage/resistance/current....when current/voltage/resistance increase what happens to the power....
V=IR voltage is directly proportional with current when resistance remain constant,voltage is directly proportional to resistance when current is constant,
resistance is inversely proportional to current when voltage is kept constant :-
power P=VI , P=I^2R, P=V^2/R :-
IF POWER INCREASE means voltage or current is increased,AND resistance decrease when voltage is kept constant!!!
hope u got it !!!!
Yes i got it last night when i was trying to solve it !! I got this anwer but anyways thankyouayeshaK....dis one is a little different den wat we usually get....here the line tht is given in front of "scale" written over there is to be measured. the line shows tht the lenght tht u hav measured(which will be almost 28 mm) is equivalent to 0.05 mm(which is given)
so,if
0.05mm(actual)=28mm (on diagram)
then
1mm=28/0.05
=560
hope u got it!
It is written in the paper that give ur answer to 3sig figures !! It is cambridge standard !!i was doing moles questions and in one question my answer was o.oo10035 moles but in marking scheme is was 0.001 moles .. can anyone please tell me a standardized method of writing moles .. like till how many decimal places or significant figures ?
Simple, use this formula V=IR to remember facts. When Current decreases, resistance decreases. When Current Increases, resistance Increases.When current increases does voltage increase?....when resistance increase does voltage increase?...when resistance increase does current decrease?....when power increase what happens to the voltage/resistance/current....when current/voltage/resistance increase what happens to the power....
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now