- Messages
- 61
- Reaction score
- 70
- Points
- 28
i checked it.Its also not in Physics Matters nor Physics federal that's why I am confused
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
i checked it.Its also not in Physics Matters nor Physics federal that's why I am confused
Relay, bell and logic gates is there. I haven't even heard this rectifying thing before let alone seeing it in the book.Okay, i will!
But Rectifying diodes. Transistors. And all the other stuff. Relay. Bell. I have no idea what all this means. :/
Is this stuff mentioned in Federal physics?
Not in the syllabusWhat is diopter and power of a lens? and what kind of questions will be there regarding it?
Yes, because all redox reactions involve oxidation and reductionDo all redox reactions involve loss and gain of electrons?
Yes, because all redox reactions involve oxidation and reduction
H2 ------> H+1 + e- (Oxidation)But what about covalent bonding?
H2 (g) + F2 (g) --> 2HF (g)
A covalent bond is formed between these two molecules, and no gain or loss of electrons takes place.
But there is a change in the oxidation state.
17) The reaction is in equilibrium. Thus whatever we do, the reaction will do something to kind of like ... uhm 'counteract' it. If we add HCl, the backward reaction will favour, shifting the position of equilibium to the left, causing the white ppt of BiOCl to fade/dissolvehttp://maxpapers.com/syllabus-materials/chemistry-5070/attachment/5070_w13_qp_1/
Can somebody please explain 10th and 17th MCQ?
Exactly, I did C too.17) The reaction is in equilibrium. Thus whatever we do, the reaction will do something to kind of like ... uhm 'counteract' it. If we add HCl, the backward reaction will favour, shifting the position of equilibium to the left, causing the white ppt of BiOCl to fade/dissolve
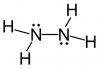
10) This is the structure of hydrazine. However, the answer is the marking scheme is given as D, but I believe it to be C
View attachment 38082
I don't know about the Chemistry Insights. My chemistry teacher gave us a lecture on it, and I had to note down the points during her lecture. Try fundamental or Chemistry matters, it could be there. It is there in the Chemistry book by Richard Harwood.Exactly, I did C too.
Where in the book can I read about this equilibrium reaction thing? Is it in Chemistry Insights?
Oh okay. I can get Chemistry Matters, though. Does it cover the whole topic?I don't know about the Chemistry Insights. My chemistry teacher gave us a lecture on it, and I had to note down the points during her lecture. Try fundamental or Chemistry matters, it could be there. It is there in the Chemistry book by Richard Harwood.
Sorry but I have no idea about the book. My teacher only told us the name of this book onceOh okay. I can get Chemistry Matters, though. Does it cover the whole topic?
Alright. Thanks anyways.Sorry but I have no idea about the book. My teacher only told us the name of this book once
17)
10) This is the structure of hydrazine. However, the answer is the marking scheme is given as D, but I believe it to be C
View attachment 38082
Exactly, I did C too.
Oh yeahThe Answer is D. The marking scheme is not wrong.
Their are 2 electrons in the first shell of Nitrogen, that are not involved in bonding, you know.
You forgot to count them?
https://docs.google.com/gview?url=h...ploads/2012/10/5070_s13_qp_12.pdf&chrome=true
Somebody please explain 19th, 20th and 31st MCQ.
Thank you.19th is A since aluminium oxides are amphoteric and carbon dioxide is acidic so they would react with NaOH. Copper and magnesium oxides don't react with bases.
20th is B since if you add more HCl then t pressure on the reactant side increases and is more than that of the product side so eq shifts forwards reducing pressure and making more Cl2
31st is D since O2 is formed at the anode and the O2 reacts with the carbon rods to give CO2. This takes place due to high temp.
no problemThank you.
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now