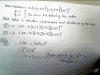- Messages
- 347
- Reaction score
- 17
- Points
- 28
guys plz help meo ut with this one...
http://www.xtremepapers.com/papers/... AS Level/Chemistry (9701)/9701_w11_qp_41.pdf
Q2a..how is it 1st order with respect to H+..im getting zero :s
http://www.xtremepapers.com/papers/... AS Level/Chemistry (9701)/9701_w11_qp_41.pdf
Q2a..how is it 1st order with respect to H+..im getting zero :s