- Messages
- 398
- Reaction score
- 761
- Points
- 73
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w09_qp_22.pdf
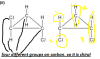
Q2 B)i)
This is regarding Diagram.. could anyone plz draw it out For Mee!
Thnx in Advance !
!
Q2 B)i)
This is regarding Diagram.. could anyone plz draw it out For Mee!
Thnx in Advance