- Messages
- 140
- Reaction score
- 134
- Points
- 38
Thankyou soo muchSorry for the crappy drawings. In my defence, drawing has never been my strength


We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Thankyou soo muchSorry for the crappy drawings. In my defence, drawing has never been my strength


thanks manFor the reaction of tollens reagent with an aldehyde the aldehyde gets converted into a carboxylic group. For the second one what you do is you remove the H2 from the 2,4 dinitrophenylhydrazine and from the other compound remove the O that is doubly bonded to the C. And then join both molecules together. The writing after the arrow is what should be written in the answer, the diagram before the arrow is for explanation.
View attachment 40925
Ekh I'm stuck with this too :/Thanks a lot, I got it now
If you don't mind, could you please help me with qn no. 3(c) of this paper? It's a similar one.. and I'm completely confused
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s11_qp_42.pdf
Can you give me the chemistry papers theory from 2000 to 2002 please?Thanx Dude!is this revision giude?
I actually started from O/n 2002!!Can you give me the chemistry papers theory from 2000 to 2002 please?
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w07_qp_32.pdf
In this paper in Q1b how should I know which experiment to follow?
Please let me know if you find the solution to itEkh I'm stuck with this too :/
Thanks a lot man, peace!w.r.t. H2O2 :
0.70/.05 = 1.4/1.0
7/5 = 7/5
w.r.t. H+ :
if we consider the conc of H2O2 first and compare it with rate:
0.09/0.05 = 1.8/1.0
9/5 = 9/5
it shows that the rate is changing just because of H2O2 .... which means that however much the conc of H+ changes, it won't have any effect on the rate of reaction. so wrt H+ the order of reaction is 0
np!! (Y)Thanks a lot man, peace!
it's a matter of the discharge priority stuff in electrlysis...Ekh I'm stuck with this too :/
hey guys
how are u all?
i need a help in http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s12_qp_23.pdf Q3 c) , Q4 c) and f) ,Q5 c) and d)
waiting your help
thanks in advance


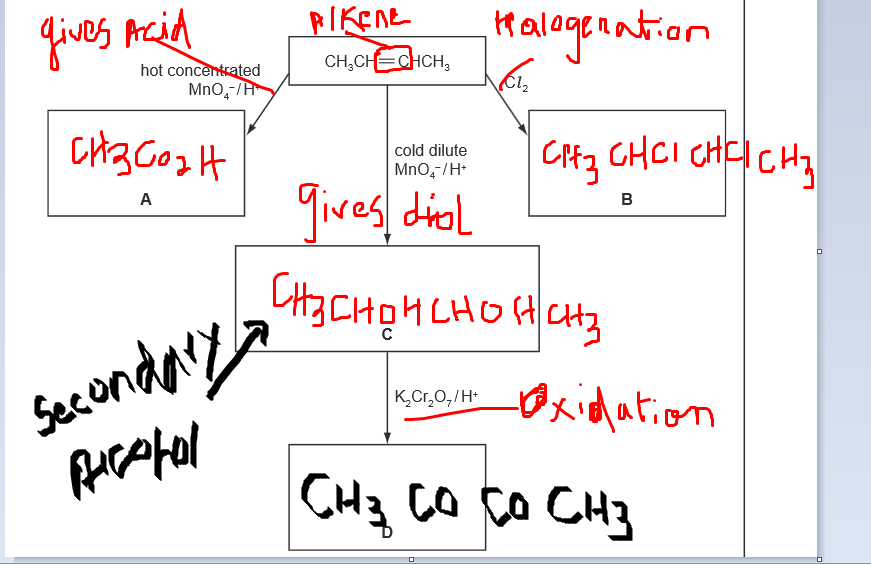

Yes,just calculate.does anyone know how to do this?
did my best to explain. dont mind my writing. i was writing in on 'paints'
Q3 c

4c

4f
5c

really thank you for your helpdont worry about the hand writing
can u plz also explain to me Q3 d) and Q 5 d)
and can u explain in more depth Q 5 C) the last step
i really appreciate your help
Wish u good luck
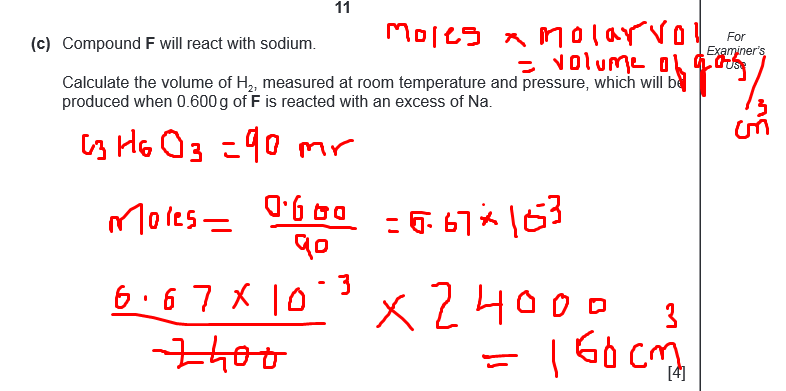
Mole ratio is 1:1
so each mole of C3H6O3 will give 1 mole of H2
so you have mass of C3H6O3 so calculate moles and multiply it by 24000 so calculate volume of gas
See, 1 Moles occupy 24 dm3 (24000 Cm3) so you can even apply the cross wala method.
1 --- --------24000
6.67*10^3 -----x
x= 160 cm3
alcohols have hydrogen bonding between O and H so that it why they are polar and Hydrogen bond is stronger to hold the liquid particle while the DME gas compound has weak vander waals
and sorry i m weak in ester isomer. need to work on it
pls do pray for me.
inshallah u will get the highest grade in all your subjects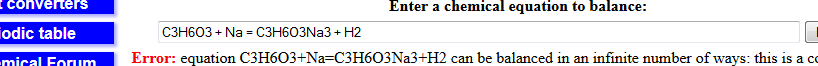
Mole ratio is 1:1
so each mole of C3H6O3 will give 1 mole of H2
so you have mass of C3H6O3 so calculate moles and multiply it by 24000 so calculate volume of gas
See, 1 Moles occupy 24 dm3 (24000 Cm3) so you can even apply the cross wala method.
1 --- --------24000
6.67*10^3 -----x
x= 160 cm3
alcohols have hydrogen bonding between O and H so that it why they are polar and Hydrogen bond is stronger to hold the liquid particle while the DME gas compound has weak vander waals
and sorry i m weak in ester isomer. need to work on it
pls do pray for me.
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now