- Messages
- 297
- Reaction score
- 696
- Points
- 93
according to the esterif u hydrolyse ester how many oh are produced
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
according to the esterif u hydrolyse ester how many oh are produced
Plz attach the link of the year and tell us the number of Q so that we can help u manhow 2 are formed is the hydroxyl group in carboxylic acid applicable also please clarify
Plz attach the link of the year and tell us the number of Q so that we can help u man
Have u seen the gt for summer and winter 2013 in both of them to get an A u need 93 marks and as u say u will lose abt 20 marks in P2 and 8 marks in P3 so that means u will get 40 in P2 and abt 32 in P3 that makes 72 so u need abt 21 marks in P1 according to last years gt to get an A so don't worry that much u will get an A inshallah and everyone else tooMan i have practiced most of P1 chemistry and still i am scoring in 20's :s
In p 21 , I think thatr i willlose 20 marks due to my silly mistakes in the silly questions
In p 32, i can lose up to 7-8 marks
so in P11,I need a high score to get an overall A incase{and for sure} the gt is high
Any way thanks man..and sure I will never lose hope...Inshallah Allah will help me
Allah kareem
Thank uHave u seen the gt for summer and winter 2013 in both of them to get an A u need 93 marks and as u say u will lose abt 20 marks in P2 and 8 marks in P3 so that means u will get 40 in P2 and abt 32 in P3 that makes 72 so u need abt 21 marks in P1 according to last years gt to get an A so don't worry that much u will get an A inshallah and everyone else too
Srry manhttp://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w09_qp_12.pdf
Q27) Ans is C Howwwwwww? Plz HElp
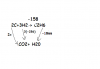
you did something wrong , the equation of reaction is PbCl4 +2NaBr--> Pbcl2+Br2+ 2NaCl.ok,
according to the data,
the equation of the reaction is.
PbCl4 +4NaBr-->Pb+2Br2+4NaCl.
now
6.980g of PbCl4
calculate its moles,Mr=349
6.980/349
=0.02mol
now since mol ration is 1:2,
we have 0.04mol of Bromine.
to get the mass.
multiply moles with its mr
= 0.04X79.9
=3.196
^ your answer.
okSrry man
Tom morning i will do the paper, So can i answer ur Q tom PLZ??!
Oct/Nov 2012 variant 11
Guys please explain question 17 the answer is D
Q 11 answers 11 answer is A
Q 10 answer is C
Question 15 answer is B
Question 4 answer is A ?? How??
Please full explanation needed!!
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w09_qp_12.pdf
Q27) Ans is C Howwwwwww? Plz HElp
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w09_qp_12.pdf
Q27 PLZ EXPLAIN WHY CANT B be the answer
Hmmmm...You have very little time left... Do u have David Ecaster,Chemistry Book... If u have Then revise maximum topics... Just try to clear as much concepts as u can... Revise ol pst paper and don't worry about the marks... Just try to clear ur concepts up... insha Allah u will do great...Man i have practiced most of P1 chemistry and still i am scoring in 20's :s
In p 21 , I think thatr i willlose 20 marks due to my silly mistakes in the silly questions
In p 32, i can lose up to 7-8 marks
so in P11,I need a high score to get an overall A incase{and for sure} the gt is high
Any way thanks man..and sure I will never lose hope...Inshallah Allah will help me
Allah kareem
U mean david Acaster. Roger Norris, Lawrie Ryan BOOK ?? Orange cover??!Hmmmm...You have very little time left... Do u have David Ecaster,Chemistry Book... If u have Then revise maximum topics... Just try to clear as much concepts as u can... Revise ol pst paper and don't worry about the marks... Just try to clear ur concepts up... insha Allah u will do great...
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w09_qp_12.pdf
Q27) Ans is C Howwwwwww? Plz HElp
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w09_qp_12.pdf
Q27 PLZ EXPLAIN WHY CANT B be the answer
In the actual ion, CH3 is attached to O from CO2 but in this arrangement it is attached to the C from CO2 so orientation is not correct and ion is different.+1 to this.
anyone, help plz.
why not B?
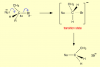
Q10:
Q10:
all the basic stuff: oxygen is more electronegative than Cl and carbon.
in C the dipoles of O and Cl will cancel a little but out to leave a smaller overall dipole. in B it is not the case. C=O means carbon has other groups bonded to it which can share it's charge. o
Q25
in the transition state the OH would have bonded to C but the Cl would have been removed -- yet. overall it will have a negative charge as OH ion had a negative ion. exampleNu represent OH ion)
View attachment 45070
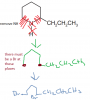
Q28
a very rough diagram:
View attachment 45071
22: In B it will form CH2OHCH2OH so H and OH are not in correct ratio and it is saturated anyway so it will not be polymerised.http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w09_qp_12.pdf
q22,
is C, y not B?
and q40.
explain plz
40: A would mean polymer has same number of molecules as monomer and would be identical to it so not possible.
If all 6.02x10^23 molecules react, it will form one molecule but 1/6.02x10^23 moles.
I'll just add on to Q40 since Ahmed Aqdam has addressed the other questions.
Q40. Requires a bit of reasoning, if 1 mole of monomer join together, we will get less than 1 mole of polymer.
If we end up with 1 mole of polymer in the end, it means none of the monomer has joined to any other!
Its like 1000 bricks (monomer) will build one house (polymer) , the number of monomer is expected to be more than polymers.
Hope it makes sense.
Q28
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now