- Messages
- 46
- Reaction score
- 28
- Points
- 28
please,may i get helped in q# 29 and 35 for may june 2013 variant 13 , answers are D and C respectively?thanks
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
When santonin is first treated with warm dilute H2SO4, and then the product of this reaction isPlz attach the link of the year and tell us the number of Q so that we can help u man
When santonin is first treated with warm dilute H2SO4, and then the product of this reaction is
treated with cold acidified KMnO4, a final product X is obtained.
How many atoms of hydrogen in each molecule of product X can be displaced with sodium
metal?
A 2 B 4 C 5 D 6
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w10_qp_11.pdf
question 29
idono why i cant solve isomer question everytime !!!! TT
An easy, albeit time consuming way to figure these questions out is to simply draw the isomers. You know there's a double bond, so it must show geometric isomerism - cis and trans, that makes 2. But they aren't asking for just geometric isomers, they're asking for all the isomers. Another one would be but-1-ene. So that makes 3. I know this isn't the best way to work it out, but it's better than nothing.
The question says 'structural or otherwise' :S I don't know, I could be wrong.
Could you or someone else help me with the questions I posted earlier? Just on the previous page.
but there is a differenc betweeen structural and stereo isomers isnt it :/?
well i think the cis and tra wont be included in this one :/
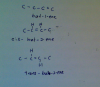
who i n helll will think to do this correct only 1 mark omg jesus christ the merciful
MJ 2008 Q27!!!some1 plz explain
so whenester bond is broken only one hydroxyl group and one cooh group is formed right then na substitutes them to get alkoxide right
5.175 then watGas X =N02
Gas Y =O2
2Mg(NO3)2 = 2MgO + 4N02 +O2
mass of gas X (NO2) produced =4*(mr of NO2)=4(46)=184
mass of gas Y (O2) produced =mr of O2=32
X/Y=184/32=5.75=1/0.174=5.75
answer = A
mass of nitrogen in 14 g = 15/100 * 14 = 2.1help urGENT PLZZZZZZZZzView attachment 45080
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now