Two equilibria are shown below.
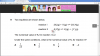
reaction I 2X2(g) + Y2(g) 2X2Y(g)
reaction II X2Y(g) X2(g) + )g( 2
1Y 2
The numerical value of Kc for reaction I is 2.
Under the same conditions, what is the numerical value of Kc for reaction II?
Answer is 1 over square root 2
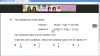
reaction I 2X2(g) + Y2(g) 2X2Y(g)
reaction II X2Y(g) X2(g) + )g( 2
1Y 2
The numerical value of Kc for reaction I is 2.
Under the same conditions, what is the numerical value of Kc for reaction II?
Answer is 1 over square root 2