- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
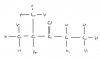
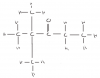
Assume there are initially 2 moles of N2O4. How many moles of N2O4 and NO2 would there be at equilibrium ?
How many moles of gas are there altogether at equilibrium ?
Can you find the partial pressures now ?
->
Initially, N2O4:NO2 is 2:0
At equilibrium, it'd be 1/3 : 2/3
Thus Kp would be (2/3)^2/ (1/3), which is 4/3!