- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
I know my abstract art. :/I was referring to the artistic style of the lone pairs you'd drawn
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
I know my abstract art. :/I was referring to the artistic style of the lone pairs you'd drawn
THANKS
SOMONE FOR HEAVENS SAKE HELP ME THROUGH THIS CRAP.http://studyguide.pk/Past Papers/CIE/International A And AS Level/9701 - Chemistry/9701_s08_qp_2.pdf
For question 5 part 3, it says F is heated with H2SO4. What I don't get is how does it change to a carboxylic acid when H2SO4 is used as a dehydrating agent? Or wait.. Only conc H2SO4 is dehydrating agent then wbu dilute? Plus for part e I know it should be an alcohol but can't be a carboxylic acid as Na also reacts with carboxylic acid to form H2? Someone HELP!
It's known as acid hydrolysis, an organic nitrile compound + mineral acid= carboxylic acid.. Organic nitrile compound + NaOH(alkali)- Sodium salt of carboxylic acid.SOMONE FOR HEAVENS SAKE HELP ME THROUGH THIS CRAP.
So, if it's an alcohol then it'll be C2(OH)H2 with double bond between C=C?It's known as acid hydrolysis, an organic nitrile compound + mineral acid= carboxylic acid.. Organic nitrile compound + NaOH(alkali)- Sodium salt of carboxylic acid.
Chapter- carboxylic acids and their derivatives.
The compound must be C2H2O2 as it's an isomer, it can't be an acid as the C would need 3 hydrogens and the MF will be C2H4O2..
If you're referring to this:http://studyguide.pk/Past Papers/CIE/International A And AS Level/9701 - Chemistry/9701_s08_qp_2.pdf
In this same paper part aii, why can't we write the structural formula like CO2H CO2H? why do we have to writeHO2CCO2H? I always get confused while writting structural formulas!SOMEONE?

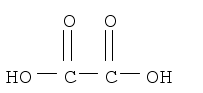
yes, dehydration reaction of an alcohol! So a double bond comes inSo, if it's an alcohol then it'll be C2(OH)H2 with double bond between C=C?
2 molecules of HOOCCOOH. The C=C bond breaks with both the C's involved in the bond being oxidised to form COOH.What is formed when HO2CCH=CHCO2H is reacted with hot conc KMNO4 ?
I hope this is helpful : http://www.docbrown.info/page07/equilibria6c.htm
Those lone pairs tho
You always seem to be panicking in your questions
A is with an alcohol so the malic acids Carboxy groups will react with them to form a diester
B is with a carboxylic acid so it'll react with the single OH present to form an ester.
Just keep these 2 things in mind and draw the structural formula like your simply making an ester from an acid and an alcohol.
Some of these questions arent that much of a problem,so i think you should at least revise the syllabus of organic a few times,and then attempt the past papers,no use just doing them without a clear concept.
Here's the mark scheme: http://freeexampapers.com/A-Level/Chemistry/CIE/2012-Jun/9701_s12_ms_22.pdf
My doubt: How do I figure out how many lone pairs are present?
Look at the groups in which Nitrogen and Sulfur reside.
Nitrogen is in group 5 and Sulfur in group 6.
That means Nitrogen has 5 electrons in its outer-shell, and Sulfur, 6.
The number of bonds an atom can form is,
No of bonds = Full valence shell - Number of valence electrons
For example, nitrogen already has 5 electrons, it needs 3 more to achieve a noble gas structure. So it will form 3 bonds.
The above formula works the same way,
No of bonds in N = 8 - 5 = 3
So with that in mind, Nitrogen can form 3 bonds and Sulfur can form 2.
The next thing to note, is that each bond will have two electrons.
Since nitrogen needs to form 3 bonds, it will need 6 electrons.
Sulfur needs to form 2 bonds, so it will need 4 electrons.
Out of the 8 electrons that can exist in the outer-shell,
-Nitrogen needs 6, which leaves 2 electrons not participating in any bond.
-Sulfur needs 4, which leaves 4 not participating in a bond.
A lone pair = 2 electrons not participating in a bond.
Nitrogen therefore has 1 lone pair and Sulfur has 2.
Hope that helped you!
Need help!!!

Q.P: http://freeexampapers.com/A-Level/Chemistry/CIE/2013-Nov/9701_w13_qp_21.pdf
M.S : http://freeexampapers.com/A-Level/Chemistry/CIE/2013-Nov/9701_w13_qp_21.pdf
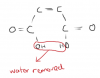
-In question 2b(ii) Do the isomers of the 3 Cyclic compounds represent cis-trans isomerism? If so, then how does cis-trans isomerism arise in cylclic compounds because I know that it occurs across double bonds of alkenes.
-And I really could not understand question 4(f).
It would be realy nice if someone could help.
And Thanks in Advance!
 ------->
-------> 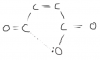 -------->
--------> 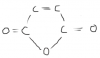
Can u plz tell me the third part as well!!There's two axes, solvent 1 and solvent 2.
If the points move across the x axis, they have separated from solvent 2.
If the points move across the y axis, they have been separated from solvent 1.
It's asking for the "two" amino acids, that did not separate from solvent 1. Which means these two points are on the same line.
View attachment 52614
They did separate from solvent 2 though.
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now