- Messages
- 1,983
- Reaction score
- 3,044
- Points
- 273
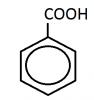
When an alkyl is attached to a benzene ring directly it would be converted into benzoic acid. no matter what that alkyl. it would always beanother thing:
when we use KMnO4 on alkyl benzene.
say we had benezene-CH2CH3.
what would be the product?
benzene-COOH or benzene-CH2COOH
also:
halogenation of phenol has 2 cases,
one where Br(l) in a non-polar solvent is used. a mixture of 2-bromo phenol and 4-bromo phenol is produced
On wikipedia it says that the reaction is faster in polar solution so in water it would be faster so there is more chance of 2,4,6. but if you use non-polar solvent and heat it and have Br2 in high concentration you can still get 2,4,6.
and one Br(aq)
2,4,6, bromo phenol produced.
is this correct?
btw.. thank you soo much Suchal Riaz
I remember you from last year bro,
congrats soo much on your distinction(saw your name in the list in feb and was soo happy for you). MashAllah!
(2) as far as I know a concentrated or heated Br2(aq) would produce 2,4,6 but milder conditions would produce just 2 or 4
(3)Thank you. If you have any other doubts please let me know.