- Messages
- 162
- Reaction score
- 249
- Points
- 53
one more question, sorry, i know i am asking too much, Do you know what the titration value is or values which are near to the correct one?then u wont probably lose sooo much marks
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
one more question, sorry, i know i am asking too much, Do you know what the titration value is or values which are near to the correct one?then u wont probably lose sooo much marks
Titration values vary centre to centre. Ask the people who performed in the same place as you for accurate readingone more question, sorry, i know i am asking too much, Do you know what the titration value is or values which are near to the correct one?
it's ok, my cousin got totally wrong, she got around 28, her teacher and classmates got around 29.4one more question, sorry, i know i am asking too much, Do you know what the titration value is or values which are near to the correct one?
They all got titration values around 29.5-29.8, i got my rough value as 29.6 and both trials 1 and 2 were 29.3 and 29.4, does this mean i am wrong? . I thought all candidates who took the same paper would be expected to get the same values. Maayee sorry for that hope he/she does well on paper 1 .Titration values vary centre to centre. Ask the people who performed in the same place as you for accurate reading
well i think u would only lose marks for accuracy but even some people did get 29.5 from ur centre and ur average titre is 29.4, so i think then it's fine... u dont need to worry that much...you will probably get good marks insha allahThey all got titration values around 29.5-29.8, i got my rough value as 29.6 and both trials 1 and 2 were 29.3 and 29.4, does this mean i am wrong? . I thought all candidates who took the same paper would be expected to get the same values. Maayee sorry for that hope he/she does well on paper 1 .
When not specified in question use the formula which shows maximum information, skelatal formulas are best for that.Do we make skeletal formulas in all structures or we can make any by choice if not specified ? Marking scheme has skeletal formulas
But does it matter if I make displayed formula ? Cause I always make mistakes in itWhen not specified in question use the formula which shows maximum information, skelatal formulas are best for that.
Then u should give all the information asked in question via displayed formulaBut does it matter if I make displayed formula ? Cause I always make mistakes in it
That would require anhydrous FeBr3 catalyst and pure bromine.Why don't we put Br to the ring with ester?? June 2014 /42
Plz help!!
Benzene does not react with bromine water, pure bromine and anyhdyrous FeBr3 is required in order for the benzene ring to react.Why don't we put Br to the ring with ester?? June 2014 /42
Plz help!!
inshallah your cousin too, thanks that made me feel better.well i think u would only lose marks for accuracy but even some people did get 29.5 from ur centre and ur average titre is 29.4, so i think then it's fine... u dont need to worry that much...you will probably get good marks insha allah
But the other Beene with OH is reacting.. I know it's a ring activator but won't the oxygen of ester activate the ring too?Benzene does not react with bromine water, pure bromine and anyhdyrous FeBr3 is required in order for the benzene ring to react.
I don't know exactly what conversation is going on so sorry to interrupt but the oxygen of the ester would not be bonded directly to the benzene ring. it would be bonded to a carbon atom which would be bonded to the ring.But the other Beene with OH is reacting.. I know it's a ring activator but won't the oxygen of ester activate the ring too?
CONFUSED
I am confused in the ring on the top which has oxygen direct,y attached to itI don't know exactly what conversation is going on so sorry to interrupt but the oxygen of the ester would not be bonded directly to the benzene ring. it would be bonded to a carbon atom which would be bonded to the ring.
you can revise the application chapter 3 from chemguideforcie.co.uk much quicker than from other places because he has given the reference to all of the latest exam questions and what was asked in them. which means you will be able to revise everything that has come in exams till now.I have a couple of questions which I was hoping somebody could help me out with.
1- Any notes on the Soil treatment(sorbents and stuff) and nano technology of applications, I got less time so something short will be appreciated.
2- I just want to confirm the conditions for ester hydrolysis. is it the same as that of hydrolysis of an amide linkage?
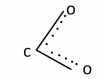
This was actually a blunder CIE made. They shouldn't have given this question because average students dont know that in COO there is a delocalisation between COO like this:I am confused in the ring on the top which has oxygen direct,y attached to it
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now