- Messages
- 1,318
- Reaction score
- 1,374
- Points
- 173
http://maxpapers.com/wp-content/uploads/2012/11/9701_w14_qp_13.pdf
Q29 ( ans is B)
30 (C) nd 20 (C) pleasee
Q20:

The green line shows the main carbon chain. These are the 5 isomers
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
http://maxpapers.com/wp-content/uploads/2012/11/9701_w14_qp_13.pdf
Q29 ( ans is B)
30 (C) nd 20 (C) pleasee

http://maxpapers.com/wp-content/uploads/2012/11/9701_w14_qp_13.pdf
Q29 ( ans is B)
30 (C) nd 20 (C) pleasee
29 -All the carbon atoms bonded in the ring have tetrahedral structure, and are sp3 hybridised. this is because every carbon is attached to four groups. So like when you draw optical isomers, in fact, every carbon with 4 substituents have a 3 dimensional tetrahedral structure. Therefore, they cannot be on the same plane. Also, there is no Cis trans isomerism for a cycloalkene made up of less than 8 carbon atoms, since the ring constricts bond rotation. So neither students are correct.http://maxpapers.com/wp-content/uploads/2012/11/9701_w14_qp_13.pdf
Q29 ( ans is B)
30 (C) nd 20 (C) pleasee
http://maxpapers.com/wp-content/uploads/2012/11/9701_w14_qp_13.pdf
Q29 ( ans is B)
30 (C) nd 20 (C) pleasee
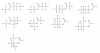
The keyword in Question 22 is "followed by". Take Option B, for example. After polymerising it, the alkene becomes saturated and therefore cannot undergo a reaction with cold, dilute KMnO4. Similarly, alkanes, which are also saturated, cannot undergo that reaction.Thanks i do understand 27 and 29 now but can you please explain 22 a bit more? thanks again
View attachment 54228
Why A? why not the others?
http://maxpapers.com/wp-content/uploads/2012/11/9701_w14_qp_13.pdf
Q19: Why is B incorrect?
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now