- Messages
- 144
- Reaction score
- 137
- Points
- 53
how do we solve this one? :/
Correct answer is C.
Since this is obviously a decomposition reaction, we can conclude that one mole of N2F4 decomposes into 2 moles of NF2. Therefore, only the N-N bond in the middle is broken, and not the N-F bonds. Option 1 is incorrect.
Option 2 states that the enthalpy change of the reaction is +160kJ/mol. This is the approximate bond enthalpy of the N-N bond, so this is correct.
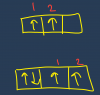
Since we have concluded that X is NF2, we can now begin to inspect it in detail. A nitrogen atom has 5 valence electrons, and 2 of these are involved in bonding. This leaves 3 other electrons in the valence shell, unbonded. Therefore, the molecule is non-linear. Option 3 is correct.
Since Options 2 and 3 are correct, the answer is C.