- Messages
- 109
- Reaction score
- 65
- Points
- 38
HEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEELP!!!PLEASE HELP ME!! I'M DYING!!!!
ANYONE??????
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
HEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEELP!!!PLEASE HELP ME!! I'M DYING!!!!
depends on how much time you've got....make sure you can finish the rest of the paper in no rush first.I have my paper 3 on 29th ... one rough titre value is enough right? or should we give 2??
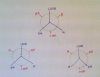
Its optical isomerism, except with 2 chiral carbons, so you need to change the position of the bonded atoms to these carbon atoms.View attachment 57308plz explain this!! thnx in advance!
is it B: 2->1->3Please please can somebody help me with this question of bond angles!
Please explain this because bond angles are so frustrating! !View attachment 57319
View attachment 57308plz explain this!! thnx in advance!
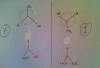
Please please can somebody help me with this question of bond angles!
Please explain this because bond angles are so frustrating! !View attachment 57319
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w13_qp_43.pdf
I have a few problems with question 6.
First one is 6 ei) How many different dipeptides is it possible to synthesise, each containing two of the three amino acids alanine, serine and lysine?
I first thought 6 (which is correct) but then i looked at the structure of lysine. it has two NH2 groups so if peptide bonds can form at either NH2 wouldn't that make it 12?? Wouldn't joining the NH2 joined to (CH2)4 would form a diff molecule if joined to the NH2 that is from the CH???
Second one is f ii) Which of the structures G, H or J is identical to structure F?
I thought it was G. But its J. I have no idea why. No matter how you flip J it doesn't look like F.
EDIT: i dont get f iii) either, why cant i just switch the positions of NH2 and COOH. that looks different
Please help and thanks in advance

View attachment 57322
How to do part b?
I understand that the values are 0=not dependant 1=proportional and 2=sqaured etc...
But I don't understand how to determine this when there are 3 different steps for 1 reaction... please explain
The answers are
1 1 0
1 1 1
1 2 2
0.05 cm3 is one drop. so u put it in estimation. usually a dropper will have a mark on it.when they ask us to add 1 cm^3 of starch indicator for titration, we add approximately how many drops?
that would come aroung like 20 drops0.05 cm3 is one drop. so u put it in estimation. usually a dropper will have a mark on it.
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now