-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 35
- Reaction score
- 18
- Points
- 18
t
 ))
))
thankyou so muchView attachment 63764
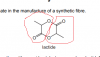
Ester breakage of the compound will help you identify it.
- Messages
- 500
- Reaction score
- 425
- Points
- 73
- Messages
- 884
- Reaction score
- 449
- Points
- 73
- Messages
- 138
- Reaction score
- 315
- Points
- 73
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
Alkenes --> diols (adding 2 OH) groups
requires cold, dilute KMnO4
- Messages
- 19
- Reaction score
- 5
- Points
- 3
- Messages
- 884
- Reaction score
- 449
- Points
- 73
Is it C?View attachment 63773
can someone help me with this question?
- Messages
- 138
- Reaction score
- 315
- Points
- 73
- Messages
- 500
- Reaction score
- 425
- Points
- 73
^ Here I have solved this question before.
I had the same problem in this question when I solved this paper but now after repeated tries I was able to solve it correctly Alhamdulillah.
First find the value of the constant K as the angle of deflection is directly proportional to the charge mass ratio
PROTON:
Angle=+15 Charge= + mass= 1
15= k(+/1) thus K=+15
Option 1 :
Charge= +1-2= -1
mass= (atomic mass) 1+2=3
k=+15
Angle= (+15)*(-1/3)= -5..............option 1 correct
Option 2 :
Charge = +3-5 = -2
mass=3+3 =6
K=+15
Angle = (+15)*(-2/6) = -5..................option 2 is also correct
Option 3 :
Charge :4-1 = +3
mass=4+5 =9
K=+15
Angle = (+15)*(+3/9) = +5.......................Option 3 is incorrect.
Ans: B
- Messages
- 138
- Reaction score
- 315
- Points
- 73
Thank you :O
- Messages
- 500
- Reaction score
- 425
- Points
- 73
Thank you :O
I am glad it helped.
- Messages
- 138
- Reaction score
- 315
- Points
- 73
- Messages
- 138
- Reaction score
- 315
- Points
- 73
- Messages
- 884
- Reaction score
- 449
- Points
- 73
- Messages
- 138
- Reaction score
- 315
- Points
- 73
Yes
- Messages
- 328
- Reaction score
- 84
- Points
- 38
- Messages
- 310
- Reaction score
- 125
- Points
- 53
Its B, since yellow ppt is formed by silver iodide.Need helpView attachment 63782
and Ba because group 2 sulfates solubility decreases down the group so Ba is less soluble than Mg and will form a precipitate.
- Messages
- 2
- Reaction score
- 0
- Points
- 11
If you find the ratio of salt to sulphite( simply by finding moles of salt and sulphite according to the info given) it comes out to be 2:1
& the equation shows 1 mole of sulphite looses 2 electron, therefore 2 mol of metallic salt gain those 2 electrons or 1 mol of metallic salt gains 1 mol of electron
therefore new O.N. of metal = +3-1=+2!
so if metallic salt gains those 2 electrons so why is +3 subtracted by -1 and not with -2.
- Messages
- 884
- Reaction score
- 449
- Points
- 73