-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 310
- Reaction score
- 125
- Points
- 53
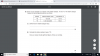
View attachment 64621 Need help with (ii)
Atomic mass is the weighted average of isotopic masses:
10.8 = 19.78%(10.0129) + 80.22%(m)
m = 10.9941 (6sf)
- Messages
- 310
- Reaction score
- 125
- Points
- 53
How did you ended up with 10.8?Atomic mass is the weighted average of isotopic masses:
10.8 = 19.78%(10.0129) + 80.22%(m)
m = 10.9941 (6sf)
- Messages
- 138
- Reaction score
- 315
- Points
- 73
Atomic weight from periodic table.How did you ended up with 10.8?
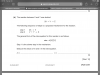
Please explain iiView attachment 64625
Few things to note:
All structural isomers have the same molecular weight of 266.5
Only the chloride ions not bonded to to Cr react (the ones outside the brackets)
n(AgCl) = n(chloride ions)
So, the number of moles of chloride ions outside the brackets is the same as the number of moles of AgCl
For A:
1g of isomer reacts with 3.75x10^-3 mol of AgCl
1g of isomer has 3.75x10^-3 mol of Cl^-1
(1/266.5) mol of isomer has 3.75x10^-3 mol of Cl^-1
3.75x10^-3 mol of isomer has 3.75x10^-3 mol of Cl^-1
This gives us a mole ratio of 1:1 between the isomer and the chloride ions outside the brackets
Therefore, 3-n = 1
n = 2
A is [Cr(H2O)4Cl2]Cl.2H2O
Same reasoning gives n value and molecular formula for B and C.
- Messages
- 310
- Reaction score
- 125
- Points
- 53
There are some qs where its of 6 marks the best thing you could for it is Practice, Practice actually helps alot.Having problem/confusion in deducing molecule thorugh NMR pattern.
Taking alot alot of time and it is of only 1-2 or maximum 3 marks.
what should i do not getting better in it?How you people doing?
- Messages
- 37
- Reaction score
- 6
- Points
- 8
does anyone have the 2019 march paper4?
- Messages
- 35
- Reaction score
- 0
- Points
- 16
- Messages
- 35
- Reaction score
- 0
- Points
- 16
The rate equation depends on the slow step in the mechanism. Here step one is the slow and it needs no Y atoms and two X atoms.Also for this one , why is N zero order, why not first? Thanks a lot in advance
For step 4: why is the reagent aqueous Hcl and heat? We are turning amide to amine so shouldn’t it be reduction with LiAlH4?
There is three ways to turn an amide to an amine
1) Acid hydrolysis, using HCL(aq) and heat
This breaks the amide linkage completely and you end up with two molecules, the amine and the acid.
The amine formed will react with the acid to form a salt.
2) Alkaline hydrolysis, using NaOH(aq) and heat
Same as acid, except the amine doesn't form salt, but the acid does.
3) Reduction, using LiAlH4
Here you end up with one molecule, an amine, the carbons in the carboxylic acid group remain attached while the oxygen is removed.
Last edited:
https://www.facebook.com/463818014075291/posts/671605593296531/?sfnsn=modoes anyone have the 2019 march paper4?
- Messages
- 310
- Reaction score
- 125
- Points
- 53
But the amine will react with the acid and form ammonium ion in acidic hydrolysisThere is two ways to turn an amide to an amine
1) Hydrolysis, using HCL(aq) and heat
This breaks the amide linkage completely and you end up with two molecules, the amine and the acid
2) Reduction, using LiAlH4
Here you end up with one molecule, an amine, the carbons in the carboxylic acid group remain attached while the oxygen is removed
- Messages
- 310
- Reaction score
- 125
- Points
- 53
I think its supposed to be NaOH(aq) + HeatFor step 4: why is the reagent aqueous Hcl and heat? We are turning amide to amine so shouldn’t it be reduction with LiAlH4?
Fair enough, edited.But the amine will react with the acid and form ammonium ion in acidic hydrolysis
- Messages
- 35
- Reaction score
- 0
- Points
- 16
For this compound Q, if we add excess Bromine , why does bromine get added to the right end side? I thought it was only on the benzene ring but in the marking scheme they said bromine is added on benzene and on the right end side
Attachments
It's electrophilic addition of bromine across the double bond.For this compound Q, if we add excess Bromine , why does bromine get added to the right end side? I thought it was only on the benzene ring but in the marking scheme they said bromine is added on benzene and on the right end side