- Messages
- 100
- Reaction score
- 185
- Points
- 53
thank u very muchSnow Angel
View attachment 40897
Question number 4 b
As a secondary cation is more stable, the first reaction more often takes place.
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
thank u very muchSnow Angel
View attachment 40897
Question number 4 b
As a secondary cation is more stable, the first reaction more often takes place.
oh thnxBasically because it has a week metallic bond. The mercury atoms doesn´t donate as much of their valence electrons to the cloud of electrons, so the electrostatic forces between the mercury cation and the delocalised sea of electrons isn´t as a strong metallic bond as say in magnesium, where there are more delocalised electrons and therefore more electrostatic forces of attraction.
ok thank uIt's actually because there are relatively weaker electrostatic forces between its positive ions and its sea of electro s by Snowy Angel!!
Haha.We always help each other,dont we...You just saved my life!!!!*happy tears*

PLZ EXPLAIN IN DETAILView attachment 40903 View attachment 40904
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w09_qp_22.pdf
Q2 B)i)
This is regarding Diagram.. could anyone plz draw it out For Mee!
Thnx in Advance!
The product will become the reactant and Reactant be product..If exo than Ea will be high for reversible and if endo than Ea be lowHow can we draw the reaction pathway for a reversible reaction :/
Thanks ^___^The product will become the reactant and Reactant be product..If exo than Ea will be high for reversible and if endo than Ea be low
Think how a reversible reaction look like for endo and exo and just make the Opp of itThanks ^___^
Thanks a lot, I got it nowAt cathode always reduction takes place
At the anode always oxidation takes place
So the object to be plated is at the cathode & it will gain the electrons from copper
You can remember it this way if it helps:
CCR- Cathode cation reduction & RIG- reduction is gain
AAO- Anode anion oxidation & OIL- oxidation is loss
Thanks a lotSimple.
Since it is an Alcohol,
When you add an oxidising agent such as K2Cr2O7 In Primary Alcohol ( OH on the end of the carbon Chain) Attached so you get an Carboxylic acid Or If its an Secondary Alcohol( OH wala Carbon attached to other two Carbon) so you get a ketone .
Presence of an Acid and Alcohol gives you an Ester. Memorize the structure of a ester. Add the Carboxylic chain on Front and Alcohol chain on the end.
When in presence of Hydrogen Hallide.. OH on gerian-ol ,The OH will be replaces by Br
Dont think how hard the name is . They make you feel like that but Just think which Functional group it contains and if this particular functional reacts with this reagent what will be formed. List down all the reaction and reagents on a page this will help u alot and will be easy to memorized .
View attachment 40878
Serious question people!! Please help. May Allah bless you all.
This stuff isn't in the syllabus or taught. This is precisely why it has shown you the 4 figures above the actual question. The examiner wants you to analyze those figures, see whats going on, and apply THAT to the question.
The question shows that all carbons in Benzene can be made to lie on the same plane, but not in cyclohexane. The question also shows that butane Carbons can be made to lie on the same plane, but methylpropane, also an alkane, cannot.
This tells us that if a compound has a ring, OR branching, it cannot be made to lie on the same plane.
All of them are co-planer apart from B.
C is coplaner because you don't have to consider the Oxygen atoms. The others are unbranched and two individual chains lying on the same plane.
ON09 42 Q5a.
I've answered this before a day or two ago but I'll do it again anyway.
Look. This stuff isn't in the syllabus or taught. This is precisely why it has shown you the 4 figures above the actual question.... lying on the same plane.
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w05_qp_2.pdf
Question 5 f) With explanations
Thanx Dude!
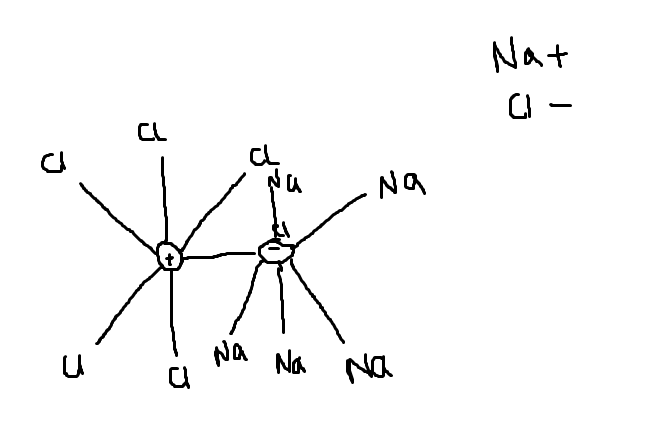
Each Na Connected to six Cl.Copied this from Endorsed Cambridge book.
*edit. Here is the image

Somebody please draw 2px 2py 2pz orbitals nd also how to show orbital s s overlap nd sp overlap
Suchal Riaz


For the reaction of tollens reagent with an aldehyde the aldehyde gets converted into a carboxylic group. For the second one what you do is you remove the H2 from the 2,4 dinitrophenylhydrazine and from the other compound remove the O that is doubly bonded to the C. And then join both molecules together. The writing after the arrow is what should be written in the answer, the diagram before the arrow is for explanation.
View attachment 40925
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now