- Messages
- 176
- Reaction score
- 186
- Points
- 53
Oh its basically like glass lid, I mean this is how I usually draw it, without knowing that tis actually existDivided flask is like this :usually used to separate SOLID reactant from the LIQUID reactant. Used in those experiments for example: CaCO3 reacting with HCl etc
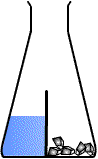
But yah thanks
